Chemistry Chapter 19 Acids Bases and Salts Section

Chemistry Chapter 19: Acids, Bases, and Salts Section 1: Acid-Base Theories

Upon completion of this lesson and homework, student will be able to: • List properties of acids and bases; • Relate the characteristics of acids and bases to [H+] and [OH-]; • Identify conjugate acid-base pairs; • Explain how p. H relates to [H+]; and • Describe how the p. H scale relates to acidity or alkalinity.

Properties of acids and bases • Acids (Acidic Solutions) – Taste sour – Can be strong or weak electrolytes in aqueous solution – Change the color of an acid-base indicator I’m basic! • Bases (Alkaline Solutions) I’m acidic! – Taste bitter – Feel slippery – can be strong or weak electrolytes in aqueous solution – Change the color of an acid-base indicator

What makes an acid? • Acids are substances that add H+ to solution – Examples: HCl, HNO 3, HF • H+ is a proton (H that lost electron) • H+ can attach to H 2 O, making H 3 O+

What makes a base? • Bases add OH- to solution • Two ways that happens: Ionic compounds with OH– anion – Ex. : Na. OH (s) → Na+(aq) + OH–(aq) Compounds that pull H+ off of H 2 O, leaving OH– – Example: NH 3

Summarizing… Which is which? H+ OH-

Conjugate Acids and Bases Conjugate acid-base pair: two substances that differ only by the presence of H+ The conjugate is the “other half”

How good are acids and bases at this proton trading? • Strong acids completely dissociate in water – They lose all their protons – Their conjugate bases don’t attract protons, so they are quite weak (not considered a base) • Weak acids only dissociate partially in water – Their conjugate bases can attract protons – Conjugates are weak bases
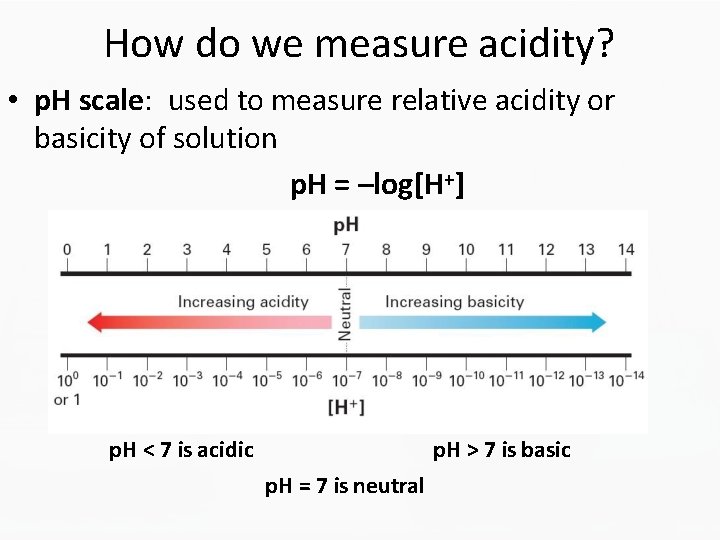
How do we measure acidity? • p. H scale: used to measure relative acidity or basicity of solution p. H = –log[H+] p. H < 7 is acidic p. H > 7 is basic p. H = 7 is neutral


Objective check: Can you. . . • List properties of acids and bases? • Relate the characteristics of acids and bases to [H+] and [OH–]? • Identify conjugate acid-base pairs? • Explain how p. H relates to [H+]? • Describe how the p. H scale relates to acidity or alkalinity?

Chemistry Chapter 19: Acids, Bases, and Salts Section 2: Hydrogen Ions and Acidity

Upon completion of this lesson and homework, student will be able to: Explain the autoionization of water; Explain how p. H relates to [H+]; Calculate p. H when given [H+]; Calculate and interpret values of [H+], [OH–] and p. H to determine if a substance is an acid or a base; and • Use acid-base indicators to estimate p. H of all kinds of stuff. • •

Water, H+ and OH– • “Pure” water isn’t all H 2 O molecules Autoionization: the splitting of water molecules into ions • Only happens with a very small fraction of water molecules [H+][OH-] = 1. 0 x 10 -14 brackets mean concentration in mol/L
![Come on, lucky sevens! • In a neutral solution, [H+] and [OH–] are balanced Come on, lucky sevens! • In a neutral solution, [H+] and [OH–] are balanced](http://slidetodoc.com/presentation_image_h2/1f89909a0d2f959d88868e5ea75e0c5d/image-15.jpg)
Come on, lucky sevens! • In a neutral solution, [H+] and [OH–] are balanced (equal) • Since [H+][OH-] = 1. 0 x 10 -14, • [H+] = [OH–] = • Acids tip the scales toward more [H+], bases toward [OH–] • When one goes up, the other must go down


Those exponents are a pain How do we scale exponents to nicer numbers? log(10 x) = x p. H = –log [H+] In pure water, p. H = −log (1. 0 10− 7) = 7. 00 An acid has a higher [H+] than pure water, so p. H is <7 • A base has a lower [H+] than pure water, so p. H is >7 • • •
![The “other” p scale • • p. OH = -log[OH-] Since [H+][OH-]=1 x 10− The “other” p scale • • p. OH = -log[OH-] Since [H+][OH-]=1 x 10−](http://slidetodoc.com/presentation_image_h2/1f89909a0d2f959d88868e5ea75e0c5d/image-18.jpg)
The “other” p scale • • p. OH = -log[OH-] Since [H+][OH-]=1 x 10− 14 p. H + p. OH = 14 These relationships among p. H, p. OH, [H+], and [OH-] can lead to hours of chemistry problem solving fun. . .
![Summing up… p. H = –log[H+] Acids: p. H < 7 [H+] > 1 Summing up… p. H = –log[H+] Acids: p. H < 7 [H+] > 1](http://slidetodoc.com/presentation_image_h2/1f89909a0d2f959d88868e5ea75e0c5d/image-19.jpg)
Summing up… p. H = –log[H+] Acids: p. H < 7 [H+] > 1 x 10 -7 [OH–] < 1 x 10 -7 Neutral p. H = 7 [H+]= [OH–]=10 -7 Bases: p. H > 7 [OH–] > 1 x 10 -7 [H+] < 1 x 10 -7
![[H+][OH–] = 1 x 10– 14 10–p. H = [H+] [OH–] 10–p. OH = [H+][OH–] = 1 x 10– 14 10–p. H = [H+] [OH–] 10–p. OH =](http://slidetodoc.com/presentation_image_h2/1f89909a0d2f959d88868e5ea75e0c5d/image-20.jpg)
[H+][OH–] = 1 x 10– 14 10–p. H = [H+] [OH–] 10–p. OH = [OH–] –log[H+] = p. H –log[OH–] = p. OH p. H + p. OH = 14 p. OH

Acid-Base Indicators • Indicators change color at a given p. H range


Objective check: Can you. . . Explain the autoionization of water? Explain how p. H relates to [H+]? Calculate p. H when given [H+]? Calculate and interpret values of [H+], [OH–] and p. H to determine if a substance is an acid or a base? • Use acid-base indicators to estimate p. H of all kinds of stuff? • •

Chemistry Chapter 19: Acids, Bases, and Salts Section 3: Strengths of Acids and Bases

Upon completion of this lesson and homework, student will be able to: • • Describe what defines a strong acid or base; Calculate p. H of solutions of strong acids and bases; Describe what defines weak acids and bases; Differentiate between acid strength and acid concentration.


Strong Acids • Strong acids: dissociate completely and irreversibly in aqueous solution into H+ and anion • Seven strong acids: HCl, HBr, HI, HNO 3, H 2 SO 4, HCl. O 3, and HCl. O 4. • To calculate p. H of a strong acid, use the acid’s concentration as [H+] H+ Cl-

p. H of a Strong Acid Example: What is the p. H of a 0. 10 M solution of HCl? So if you have 0. 10 M HCl, have [H+] = 0. 10 M p. H = -log(0. 10) p. H = 1. 00

Strong Bases • Strong bases: dissociate completely and irreversibly in aqueous solution into metal cations and OH • Examples: soluble hydroxides of alkali metal and heavier alkaline earth metal hydroxides (Ca 2+, Sr 2+, and Ba 2+) • To calculate p. H of a strong base, use the base’s concentration to determine [OH-] Na+ OH-

p. H of a Strong Base Example: What is the p. H of a 0. 15 M solution of Na. OH? • If you have 0. 15 M Na. OH, [OH-] = 0. 15 M p. OH = -log(0. 15) p. OH = 0. 82 p. H = 14. 00 – 0. 82 = 13. 18

p. H of a Strong Base Example 2: What is the p. H of a 0. 050 M solution of Ca(OH)2? • Ca(OH)2 dissociates completely: • Ca(OH)2 → Ca 2+ + 2 OH • So [OH-] = 2(0. 050 M) = 0. 10 M p. OH = -log(0. 10) p. OH = 1. 00 p. H = 14. 00 – 1. 00 = 13. 00

Weak acids are more complicated. . . Weak acids: don’t dissociate completely + • Most of the acid holds onto H • Reach equilibrium: HA(aq) A−(aq) + H+(aq) That’s so weak! • Equilibrium concentration of undissociated acid is much higher than concentration of H+


Weak Bases Weak bases: react with water to form the hydroxide ion and the conjugate acid of the base • Don’t bring OH−, have to make it • Like weak acids, exist in equilibrium

Weak Bases I’m weak. . . De. Long Most of the weak base doesn’t make OH−

Difference between strength and concentration • Concentration: how much acid or base in a solution (usually mol/L) • Strength: how much of substance will dissociate and make solution acidic or basic • A strong acid solution of concentration equal to a weak acid will always have a lower p. H • Same for strong and weak bases

Objective check: Can you. . . • Describe what defines a strong acid or base? • Calculate p. H of solutions of strong acids and bases? • Describe what defines weak acids and bases? • Differentiate between acid strength and acid concentration?

Chemistry Chapter 19: Acids, Bases, and Salts Section 4: Neutralization Reactions

Upon completion of this lesson and homework, student will be Define the term neutralization; able to: • • Describe the products of an acid-base reaction; and • Define the terms titration and equivalence point.


Acid-Base Reactions Neutralization: reaction of acid with base to produce water + salt Is that salt? Salt: compound with cation of base and anion of acid + = +

Titration: reaction of a solution of unknown concentration with a solution of known (standard) concentration Equivalence point: when stoichiometrically equivalent amounts of known and unknown are present For strong acid & strong base, solution is neutral at equivalence point [? ] + [0. 1 M] = p. H 7

How strong is your acid? • If you have acid of unknown concentration. . . • Add base of known concentration until neutralized • Vol. base x [base] = moles base • At equivalence point: moles base = moles acid How do I know it’s neutralized?
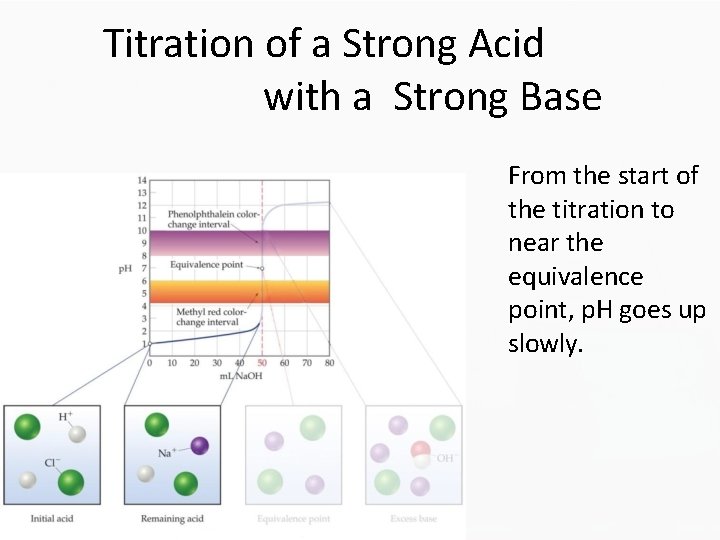
Titration of a Strong Acid with a Strong Base From the start of the titration to near the equivalence point, p. H goes up slowly.

Objective check: Can you. . . • Define the term neutralization? • Describe the products of an acid-base reaction? • Define the terms titration and equivalence point?
- Slides: 45