Chemistry Chapter 13 States of Matter Kinetic Molecular




- Slides: 47

Chemistry: Chapter 13 States of Matter

Kinetic Molecular Theory (KMT): accounts for the behavior of atoms and molecules that make up matter. KMT is based on idea that particles of matter are always in motion and upon these 5 assumptions…

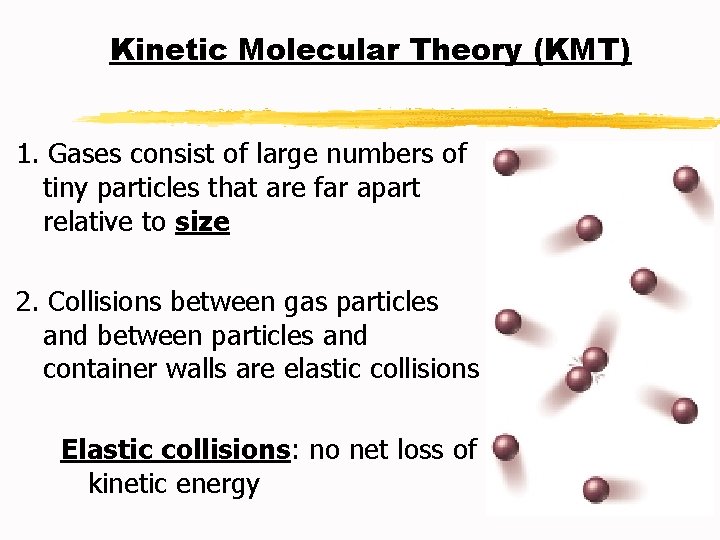
Kinetic Molecular Theory (KMT) 1. Gases consist of large numbers of tiny particles that are far apart relative to size 2. Collisions between gas particles and between particles and container walls are elastic collisions Elastic collisions: no net loss of kinetic energy

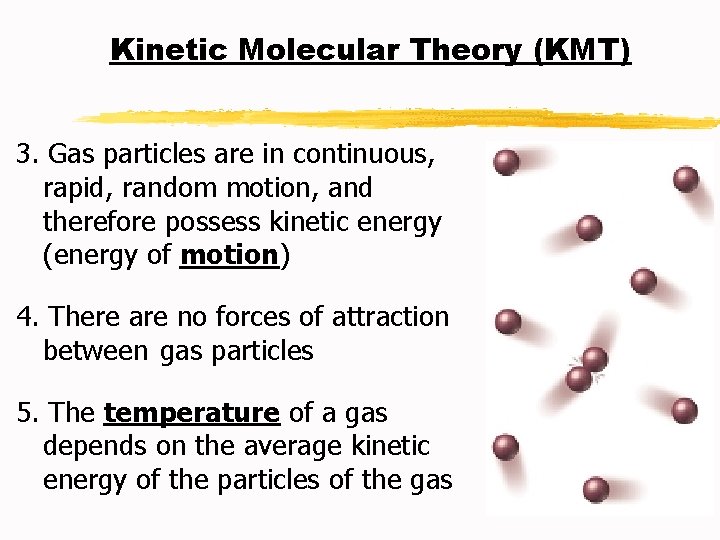
Kinetic Molecular Theory (KMT) 3. Gas particles are in continuous, rapid, random motion, and therefore possess kinetic energy (energy of motion) 4. There are no forces of attraction between gas particles 5. The temperature of a gas depends on the average kinetic energy of the particles of the gas

The Nature of Gases a. Gases have no definite shape or volume b. Gases have fluidity (the ability to flow) c. Gases have low density d. Gases have compressibility

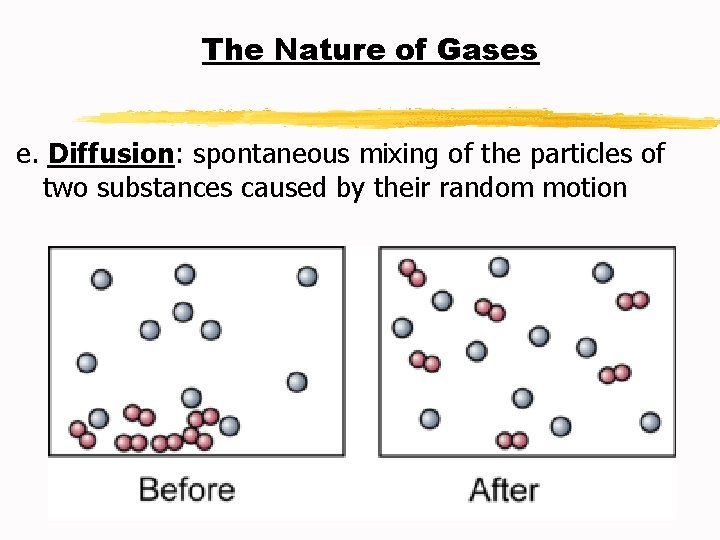
The Nature of Gases e. Diffusion: spontaneous mixing of the particles of two substances caused by their random motion

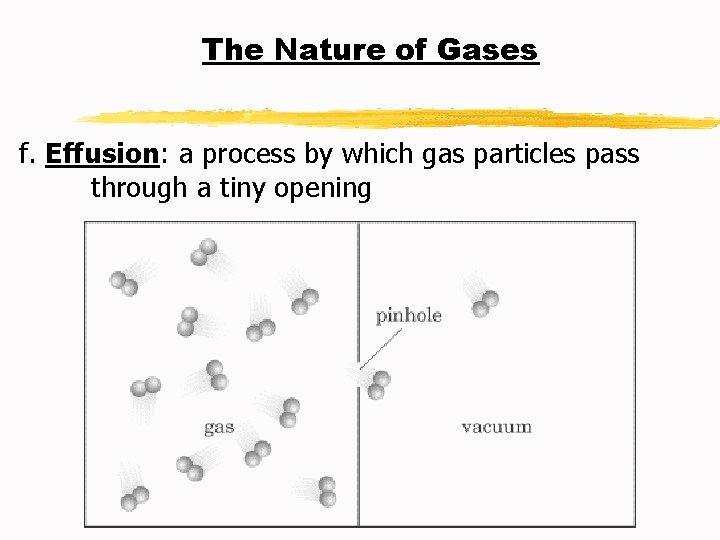
The Nature of Gases f. Effusion: a process by which gas particles pass through a tiny opening

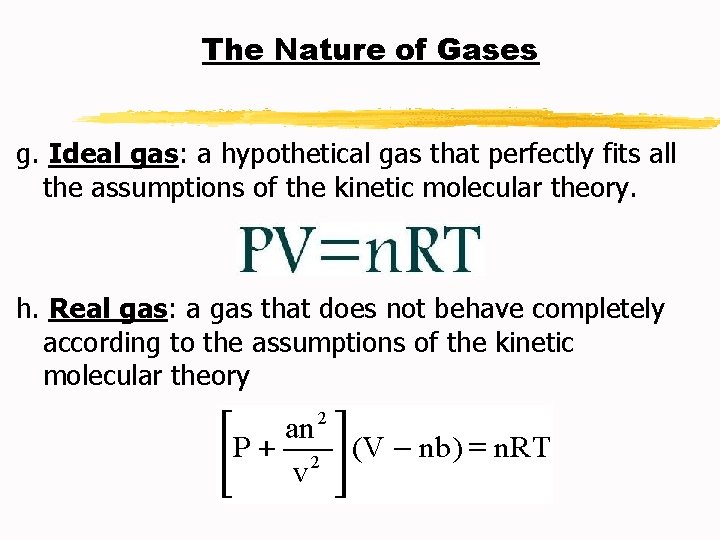
The Nature of Gases g. Ideal gas: a hypothetical gas that perfectly fits all the assumptions of the kinetic molecular theory. h. Real gas: a gas that does not behave completely according to the assumptions of the kinetic molecular theory

Properties of Liquids A. Liquids have definite volume, but no definite shape B. Relatively high density (100 s of times denser than gases) C. Relative Incompressibility D. Ability to diffuse (slower than gases)

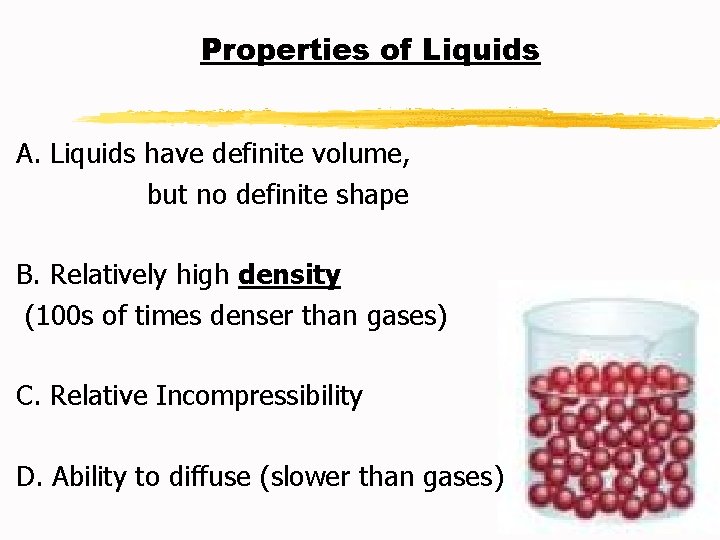
Properties of Liquids E. Surface tension: a force that tends to pull adjacent parts of a liquid’s surface togethereby decreasing surface area to the smallest possible size.

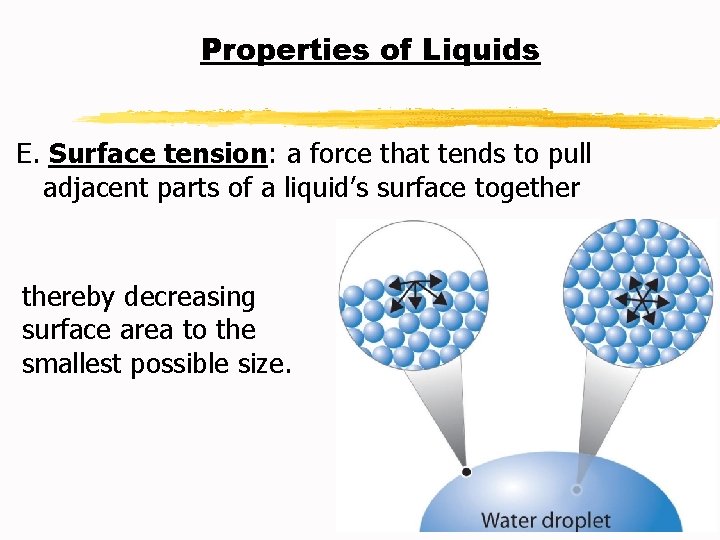
Properties of Liquids F. Capillary action: the attraction of the surface of a liquid to the surface of a solid

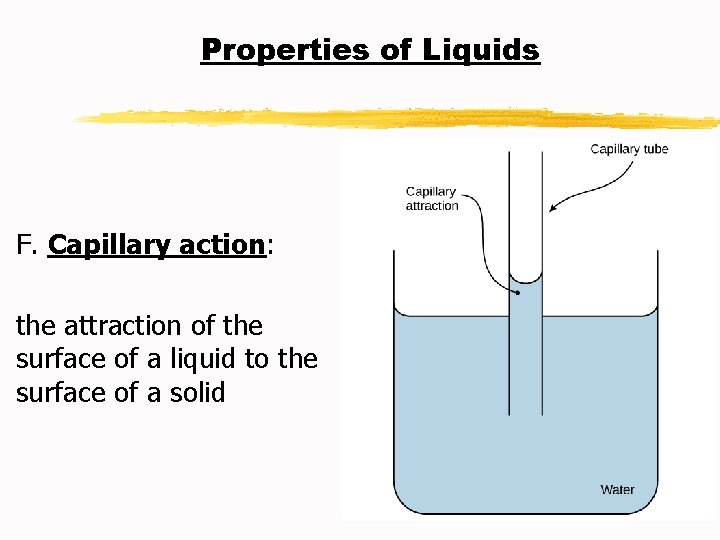
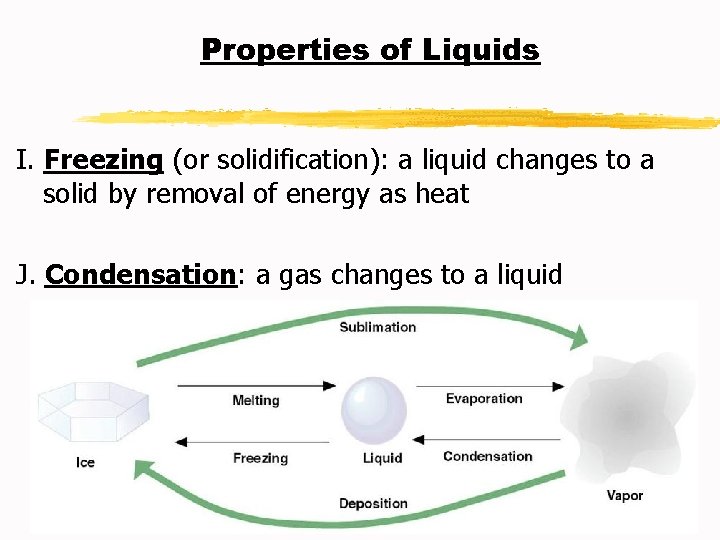
Properties of Liquids G. Vaporization: a liquid changes to a gas H. Evaporation: particles escape from the surface of a nonboiling liquid and enter the gaseous state

Properties of Liquids I. Freezing (or solidification): a liquid changes to a solid by removal of energy as heat J. Condensation: a gas changes to a liquid

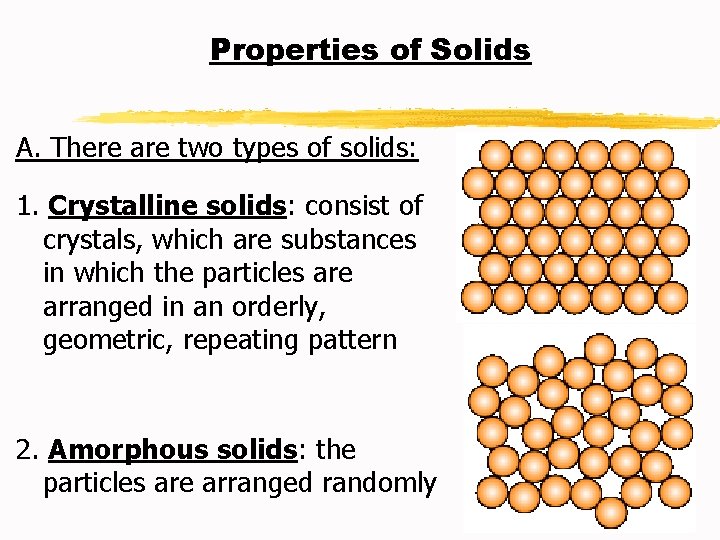
Properties of Solids A. There are two types of solids: 1. Crystalline solids: consist of crystals, which are substances in which the particles are arranged in an orderly, geometric, repeating pattern 2. Amorphous solids: the particles are arranged randomly

Properties of Solids B. Solids have a definite shape and a definite volume C. Have a definite melting point: the temperature at which a solid becomes a liquid D. Melting: a solid changes to a liquid by the addition of energy as heat

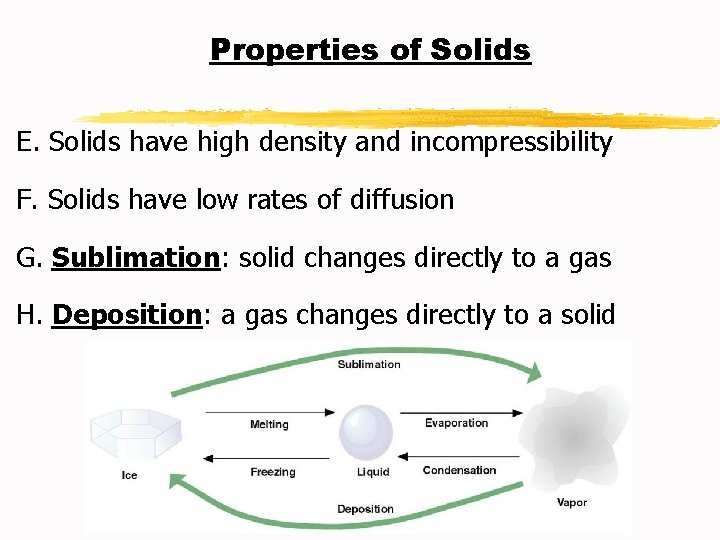
Properties of Solids E. Solids have high density and incompressibility F. Solids have low rates of diffusion G. Sublimation: solid changes directly to a gas H. Deposition: a gas changes directly to a solid

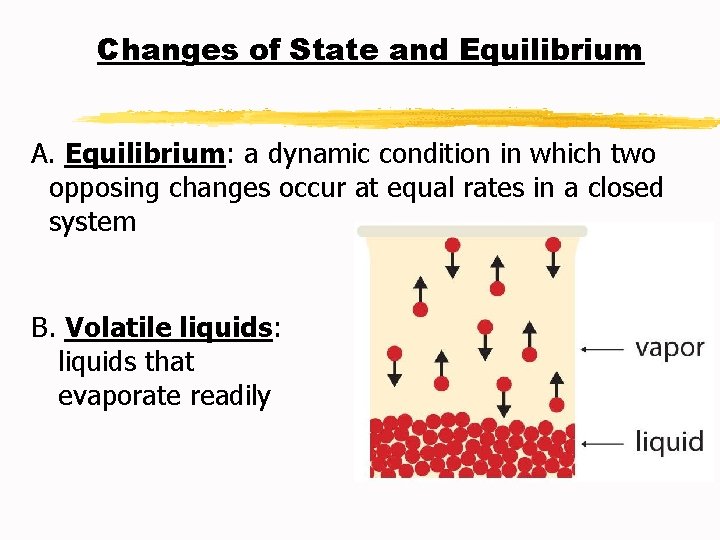
Changes of State and Equilibrium A. Equilibrium: a dynamic condition in which two opposing changes occur at equal rates in a closed system B. Volatile liquids: liquids that evaporate readily

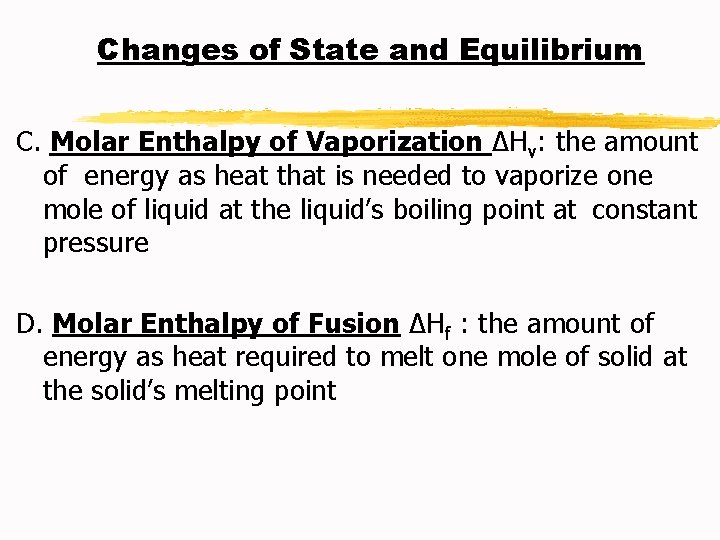
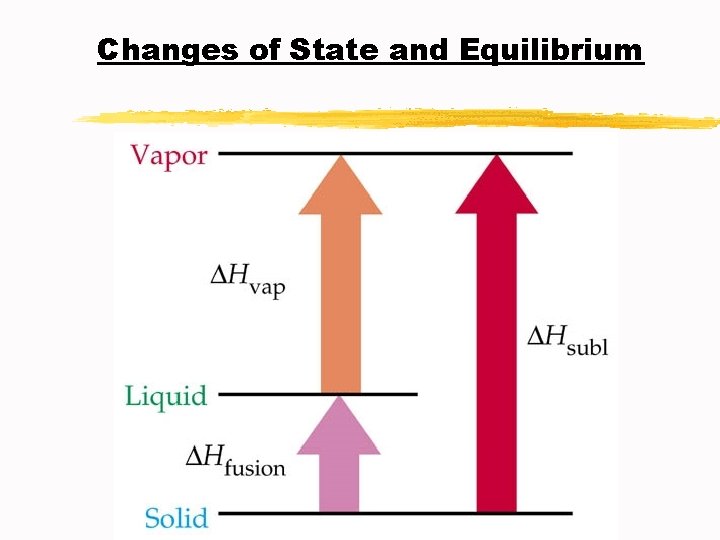
Changes of State and Equilibrium C. Molar Enthalpy of Vaporization ∆Hv: the amount of energy as heat that is needed to vaporize one mole of liquid at the liquid’s boiling point at constant pressure D. Molar Enthalpy of Fusion ∆Hf : the amount of energy as heat required to melt one mole of solid at the solid’s melting point

Changes of State and Equilibrium

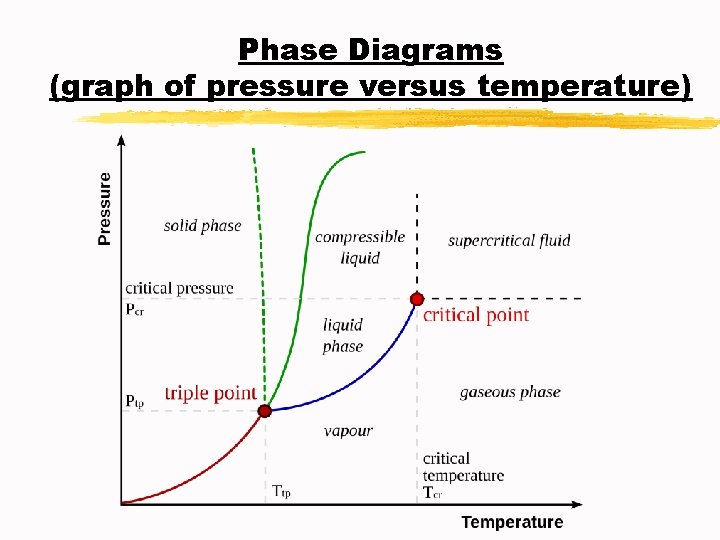
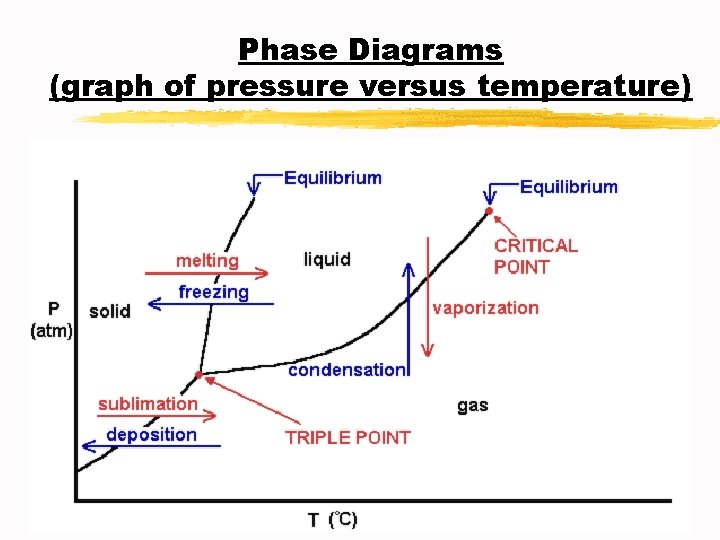
Phase Diagrams (graph of pressure versus temperature)

Phase Diagrams (graph of pressure versus temperature) A. Triple point: indicates the temperature and pressure at which the solid, liquid, and vapor of a substance coexist at equilibrium B. Critical point: indicates the critical temperature and critical pressure C. Critical temperature Tc: the temperature above which the substance cannot exist in the liquid state D. Critical pressure Pc: the lowest pressure at which the substance cannot exist as a liquid at the critical temperature

Phase Diagrams (graph of pressure versus temperature)

Chapters 15 & 16 – Solutions

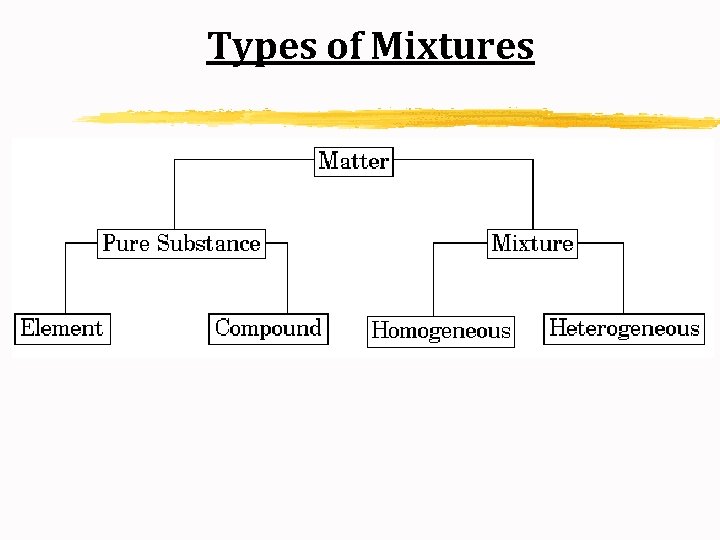
Types of Mixtures

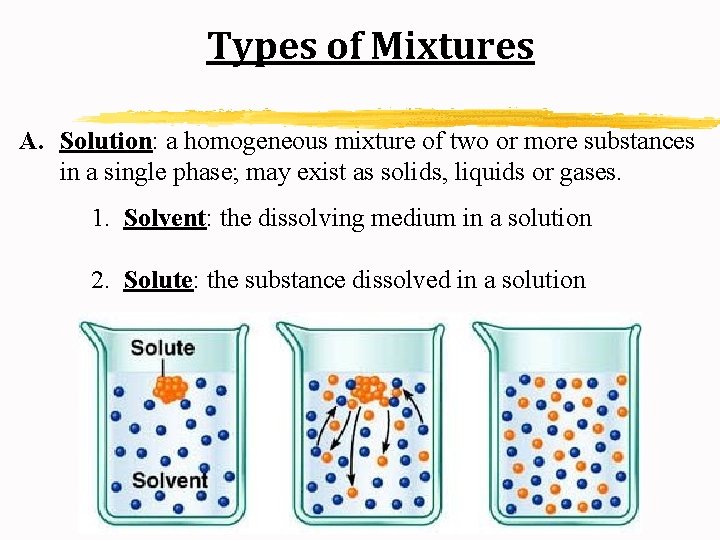
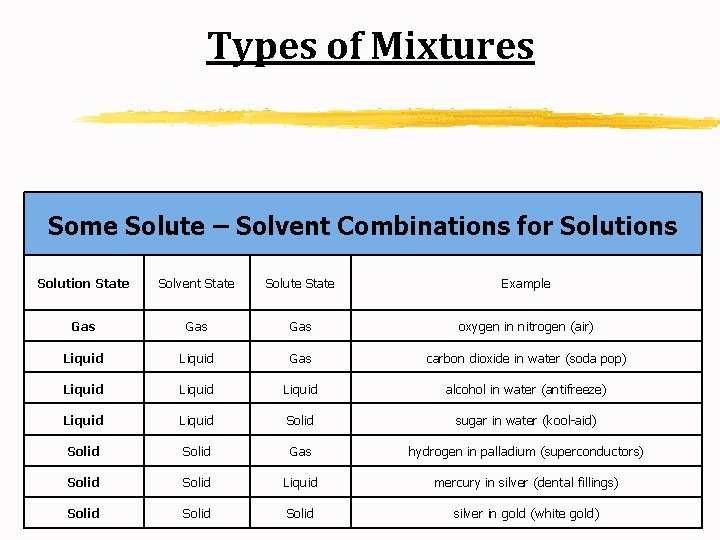
Types of Mixtures A. Solution: a homogeneous mixture of two or more substances in a single phase; may exist as solids, liquids or gases. 1. Solvent: the dissolving medium in a solution 2. Solute: the substance dissolved in a solution

Types of Mixtures Some Solute – Solvent Combinations for Solutions Solution State Solvent State Solute State Example Gas Gas oxygen in nitrogen (air) Liquid Gas carbon dioxide in water (soda pop) Liquid alcohol in water (antifreeze) Liquid Solid sugar in water (kool-aid) Solid Gas hydrogen in palladium (superconductors) Solid Liquid mercury in silver (dental fillings) Solid silver in gold (white gold)

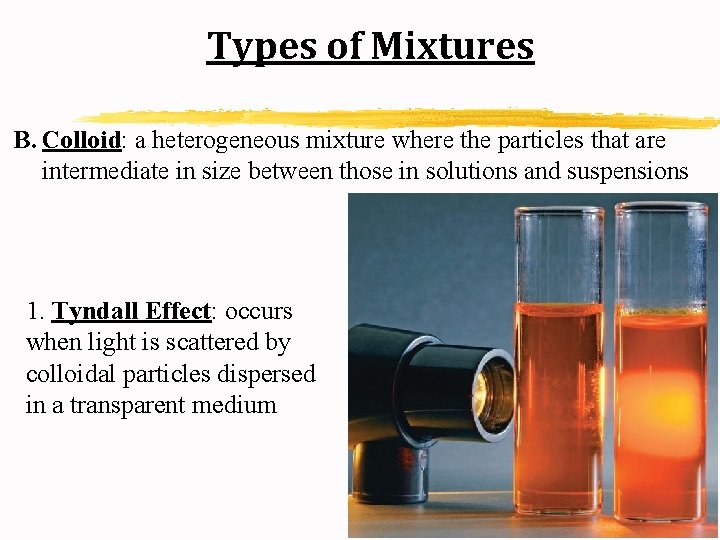
Types of Mixtures B. Colloid: a heterogeneous mixture where the particles that are intermediate in size between those in solutions and suspensions 1. Tyndall Effect: occurs when light is scattered by colloidal particles dispersed in a transparent medium

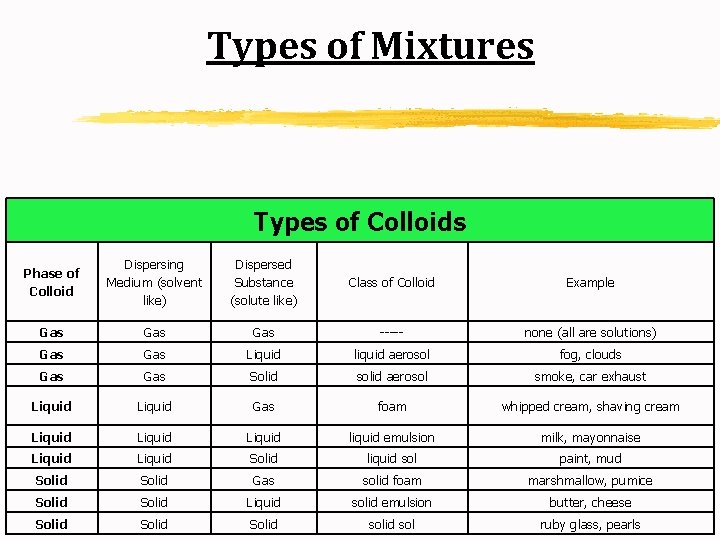
Types of Mixtures Types of Colloids Phase of Colloid Dispersing Medium (solvent like) Dispersed Substance (solute like) Class of Colloid Example Gas Gas ----- none (all are solutions) Gas Liquid liquid aerosol fog, clouds Gas Solid solid aerosol smoke, car exhaust Liquid Gas foam whipped cream, shaving cream Liquid liquid emulsion milk, mayonnaise Liquid Solid liquid sol paint, mud Solid Gas solid foam marshmallow, pumice Solid Liquid solid emulsion butter, cheese Solid sol ruby glass, pearls

Types of Mixtures C. Suspension: a heterogeneous mixture where the particles in a solvent are so large that they settle out unless the mixture is constantly stirred or agitated.

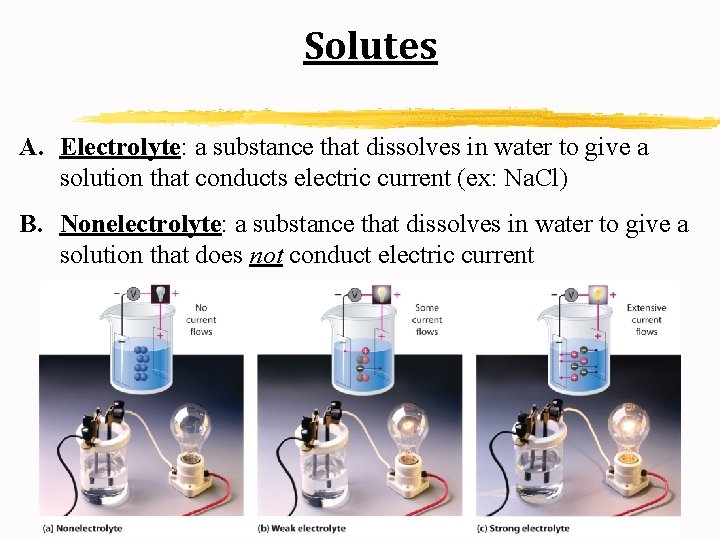
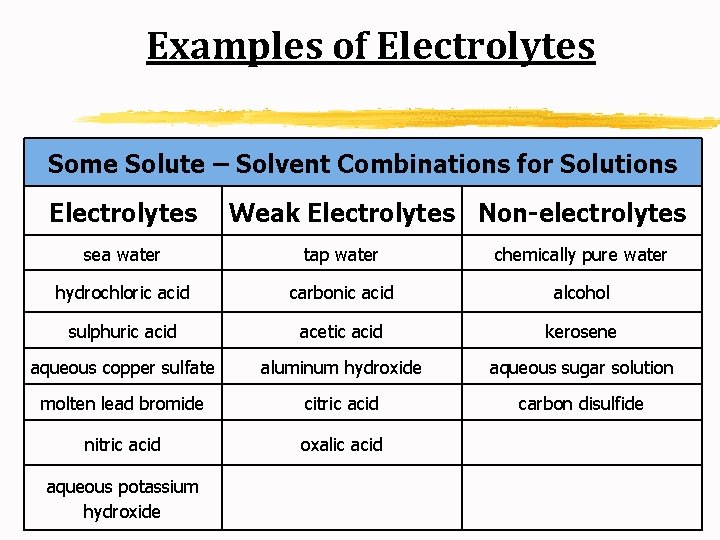
Solutes A. Electrolyte: a substance that dissolves in water to give a solution that conducts electric current (ex: Na. Cl) B. Nonelectrolyte: a substance that dissolves in water to give a solution that does not conduct electric current

Examples of Electrolytes Some Solute – Solvent Combinations for Solutions Electrolytes Weak Electrolytes Non-electrolytes sea water tap water chemically pure water hydrochloric acid carbonic acid alcohol sulphuric acid acetic acid kerosene aqueous copper sulfate aluminum hydroxide aqueous sugar solution molten lead bromide citric acid carbon disulfide nitric acid oxalic acid aqueous potassium hydroxide

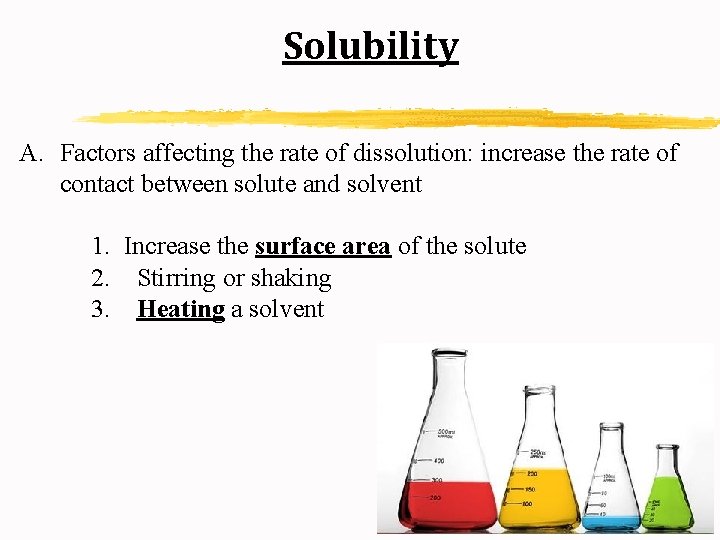
Solubility A. Factors affecting the rate of dissolution: increase the rate of contact between solute and solvent 1. Increase the surface area of the solute 2. Stirring or shaking 3. Heating a solvent

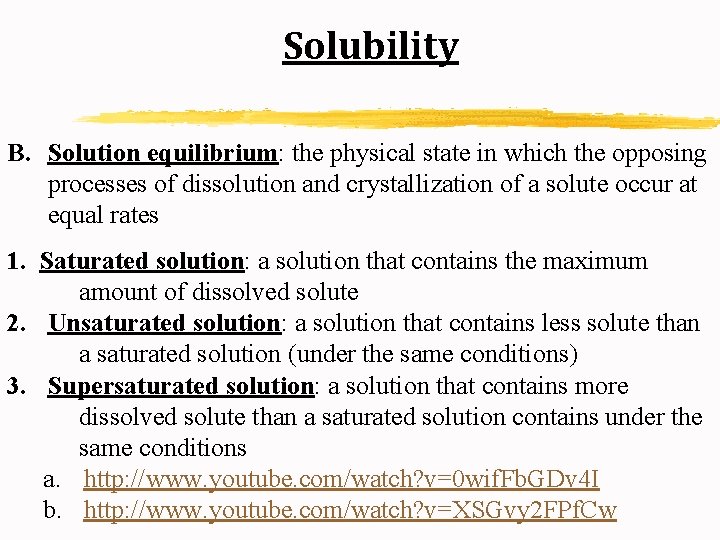
Solubility B. Solution equilibrium: the physical state in which the opposing processes of dissolution and crystallization of a solute occur at equal rates 1. Saturated solution: a solution that contains the maximum amount of dissolved solute 2. Unsaturated solution: a solution that contains less solute than a saturated solution (under the same conditions) 3. Supersaturated solution: a solution that contains more dissolved solute than a saturated solution contains under the same conditions a. http: //www. youtube. com/watch? v=0 wif. Fb. GDv 4 I b. http: //www. youtube. com/watch? v=XSGvy 2 FPf. Cw

Solubility C. Solubility: the amount of a substance required to form a saturated solution with a specific amount of solvent at a specified temperature 1. Immiscible: the term for liquids that are not soluble in each other (oil & water) 2. Miscible: the term for liquids that dissolve freely in one another in any proportion (sugar and water) 3. “like dissolves like”

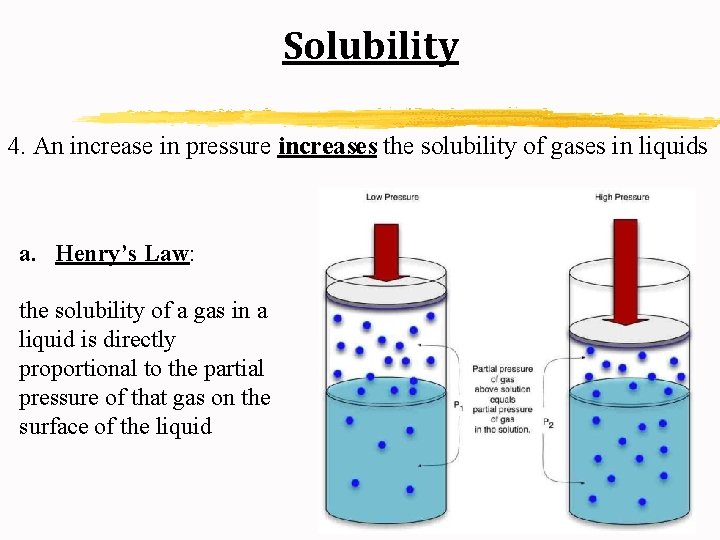
Solubility 4. An increase in pressure increases the solubility of gases in liquids a. Henry’s Law: the solubility of a gas in a liquid is directly proportional to the partial pressure of that gas on the surface of the liquid

Solubility b. effervescence: the rapid escape of a gas from a liquid in which it is dissolved

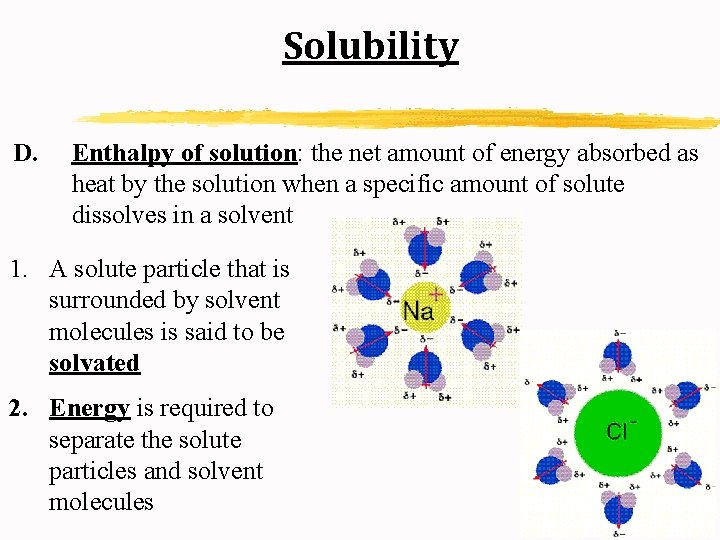
Solubility D. Enthalpy of solution: the net amount of energy absorbed as heat by the solution when a specific amount of solute dissolves in a solvent 1. A solute particle that is surrounded by solvent molecules is said to be solvated 2. Energy is required to separate the solute particles and solvent molecules

Concentration of Solutions A. Concentration: is a measure of the amount of solute in a given amount of solvent or solution

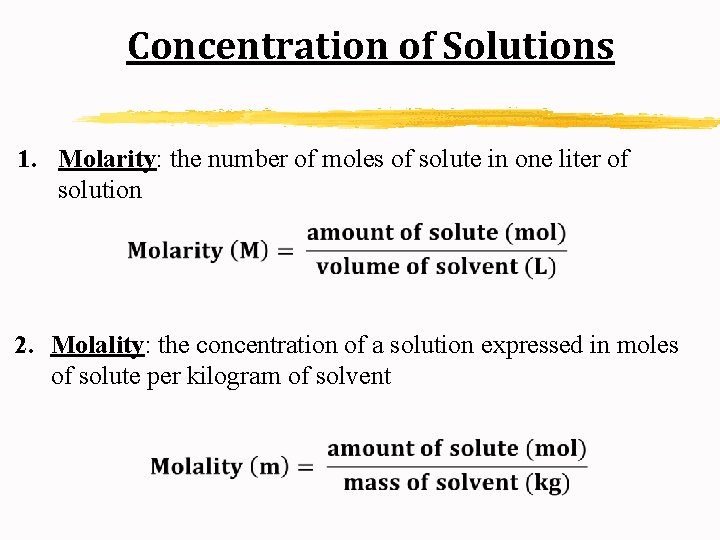
Concentration of Solutions 1. Molarity: the number of moles of solute in one liter of solution 2. Molality: the concentration of a solution expressed in moles of solute per kilogram of solvent

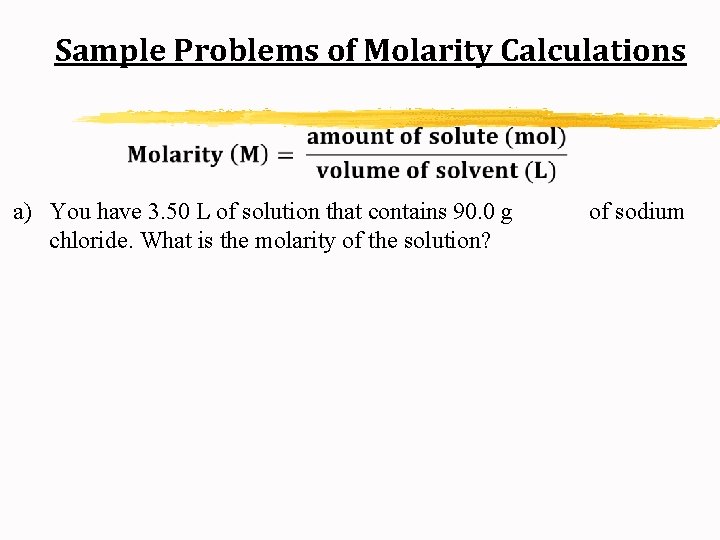
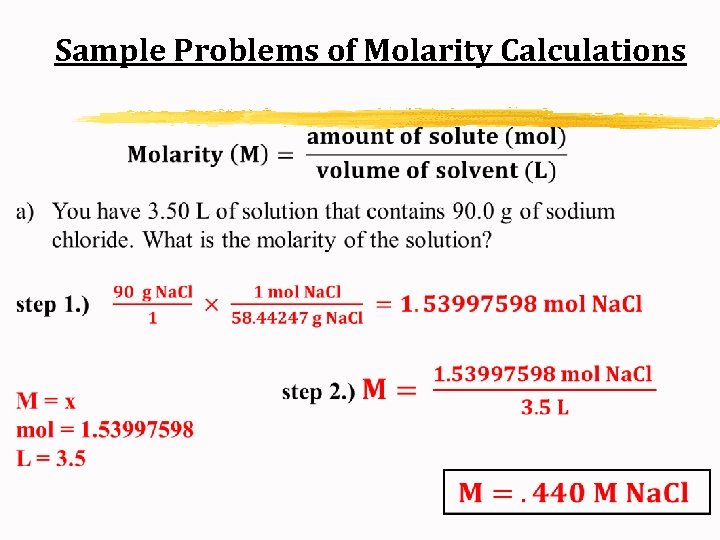
Sample Problems of Molarity Calculations a) You have 3. 50 L of solution that contains 90. 0 g chloride. What is the molarity of the solution? of sodium

Sample Problems of Molarity Calculations

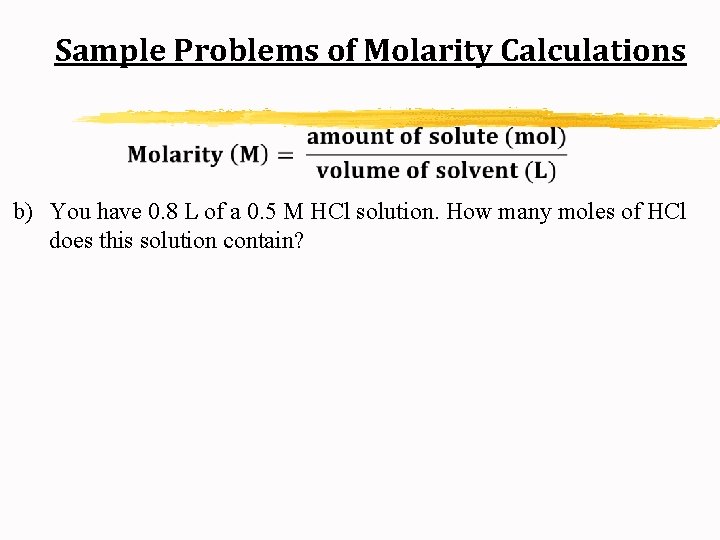
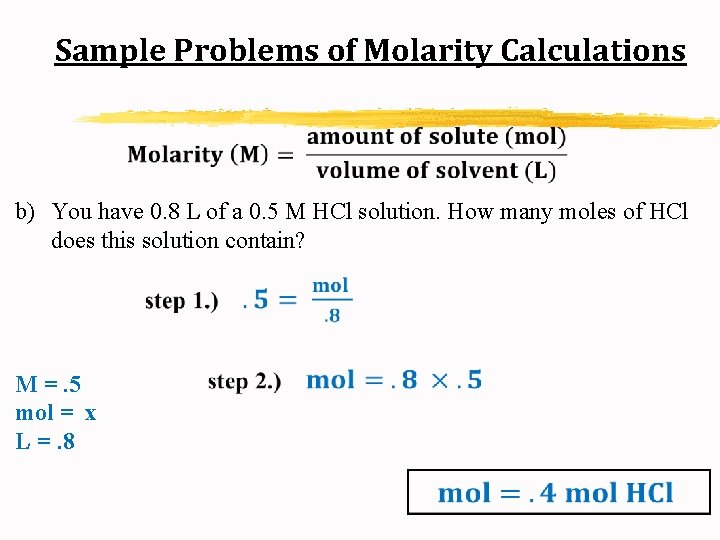
Sample Problems of Molarity Calculations b) You have 0. 8 L of a 0. 5 M HCl solution. How many moles of HCl does this solution contain?

Sample Problems of Molarity Calculations b) You have 0. 8 L of a 0. 5 M HCl solution. How many moles of HCl does this solution contain? M =. 5 mol = x L =. 8

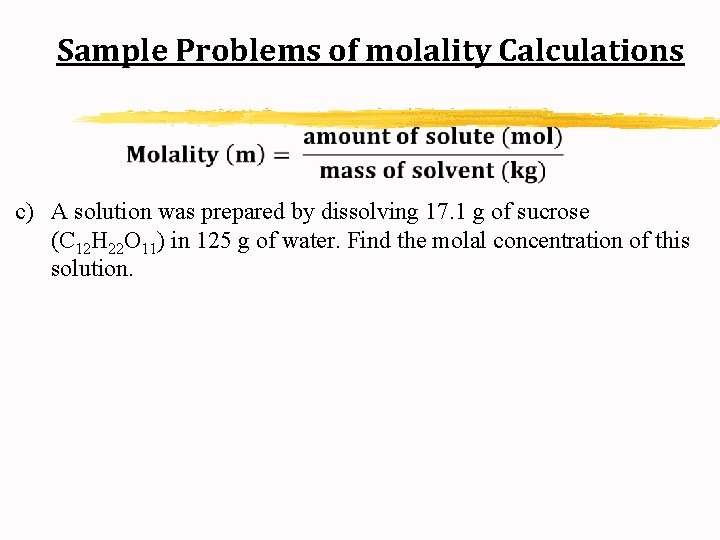
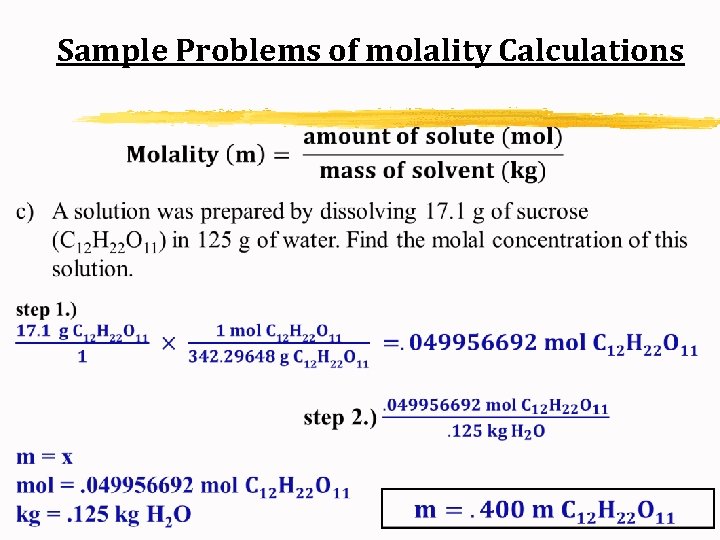
Sample Problems of molality Calculations c) A solution was prepared by dissolving 17. 1 g of sucrose (C 12 H 22 O 11) in 125 g of water. Find the molal concentration of this solution.

Sample Problems of molality Calculations

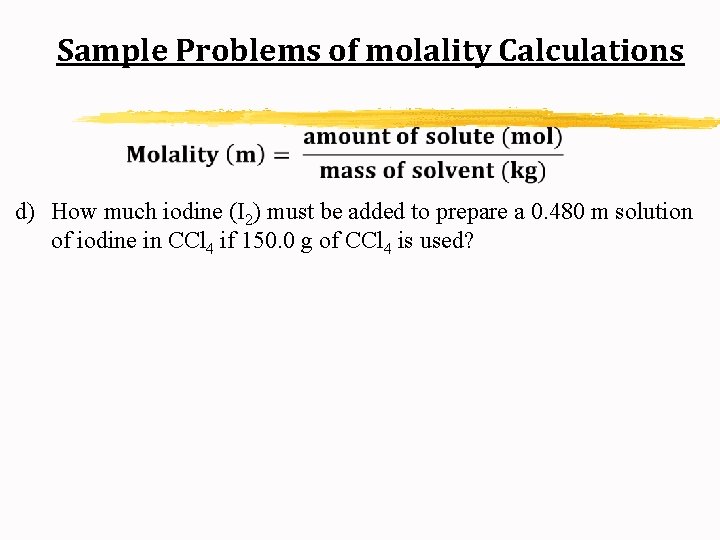
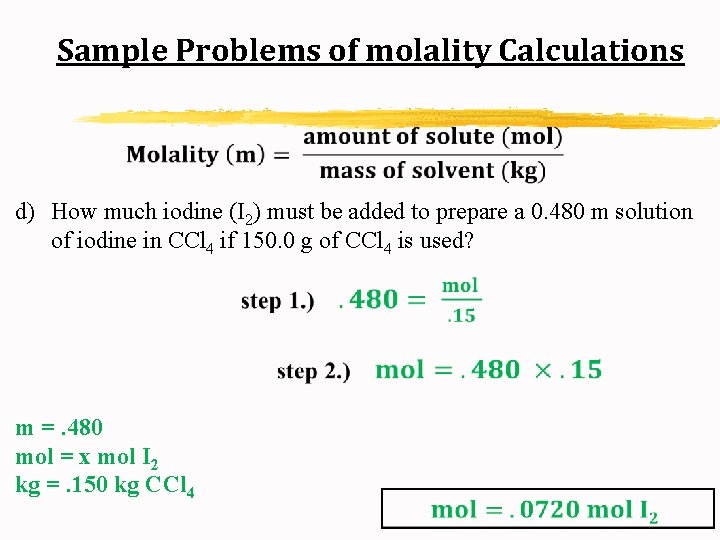
Sample Problems of molality Calculations d) How much iodine (I 2) must be added to prepare a 0. 480 m solution of iodine in CCl 4 if 150. 0 g of CCl 4 is used?

Sample Problems of molality Calculations d) How much iodine (I 2) must be added to prepare a 0. 480 m solution of iodine in CCl 4 if 150. 0 g of CCl 4 is used? m =. 480 mol = x mol I 2 kg =. 150 kg CCl 4