Chemistry Basics Elements Compounds and Mixtures Lets start

Chemistry Basics Elements, Compounds and Mixtures

Let’s start with elements • A element is a substance that can’t be broken down by ordinary chemical means. • What are those? • Heating, Cooling, Running an electric current through it

A Pure Substance • An element is a pure substance – that means it only contains one type of particle. • These particles are called atoms – we will learn more about them in Chapter 11
What kind of properties do elements have? • They have characteristic properties – who remembers what those are? • Properties that don’t depend on the amount of the element present • Examples include boiling and melting points, density, reactivity • Some elements share properties. • For example krypton and helium are both unreactive but they don’t have the same densities

Question Time • A substance that can’t be broken down by ordinary chemical means is an a. molecule b. compound c. element • All of the following are ordinary chemical means except a. cooling b. running a current through it c. heating d. pressurizing • Properties that don’t matter no matter what size or mass the substance is are said to be a. chemical b. characteristic c. physical

Identifying Elements by their Properties • In order to identify an element we have to know some of it’s properties. • In addition to its characteristic properties, we can look at color, hardness, and texture. Some elements burn, some react with water while other react with acids.

Classifying Elements • Think about dogs for a second. • We can identify them by their size, length of hair, appearance • We do the same with elements
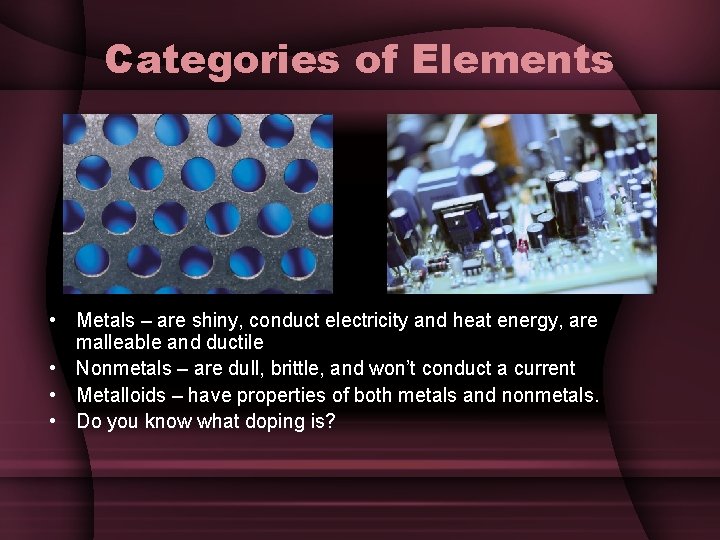
Categories of Elements • Metals – are shiny, conduct electricity and heat energy, are malleable and ductile • Nonmetals – are dull, brittle, and won’t conduct a current • Metalloids – have properties of both metals and nonmetals. • Do you know what doping is?

Categories are Similar • Think of the categories of elements as being in a music store • CD’s are broken down into jazz, soft rock, movies, blues, alternative, etc. • If you know what category an element is in you can predict some of its properties.

Question Time • 1. Elements that are shiny and conduct electricity are usually a. nonmetals b. metalloids or c. metals • 2. Elements that are usually brittle and dull are often a. metalloids b. nonmetals c. metals • 3. Adding an element to another element to make it have special properties is called a. addition b. summing c. doping d. hacking

Time to Summarize What particle scientists do for fun! • A substance in which all the particles are alike is a pure substance. • Elements are pure substances • They can’t be broken down by simple means • Every element has it’s own unique properties but share some of their properties with other elements • Some of those properties include boiling point, density, flammabiltiy, and melting point • Elements are classified as metals, nonmetals or metalloids
- Slides: 11