Chemistry Basics Elements and Atoms Element vs Compound
Chemistry Basics Elements and Atoms
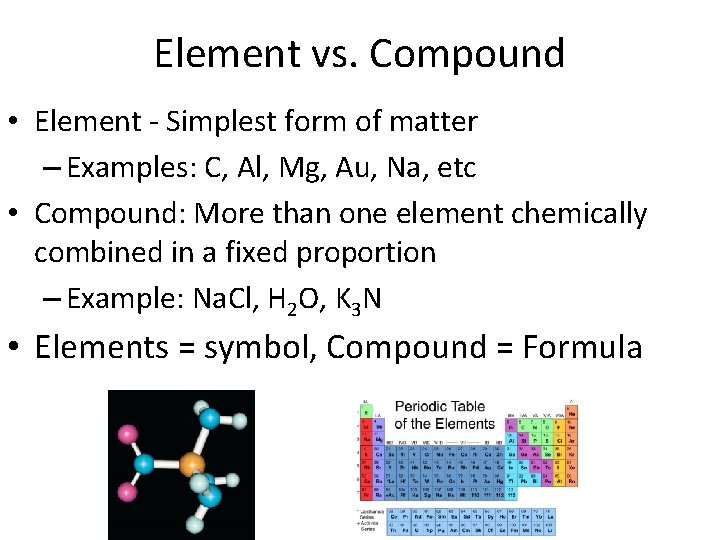
Element vs. Compound • Element - Simplest form of matter – Examples: C, Al, Mg, Au, Na, etc • Compound: More than one element chemically combined in a fixed proportion – Example: Na. Cl, H 2 O, K 3 N • Elements = symbol, Compound = Formula
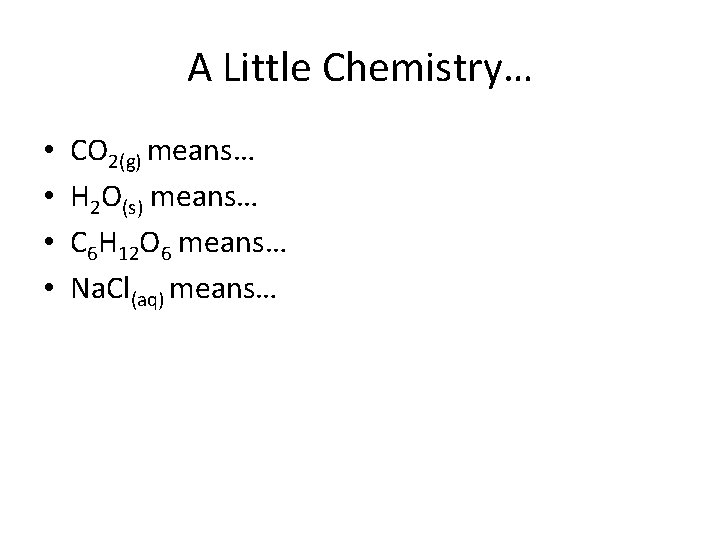
A Little Chemistry… • • CO 2(g) means… H 2 O(s) means… C 6 H 12 O 6 means… Na. Cl(aq) means…
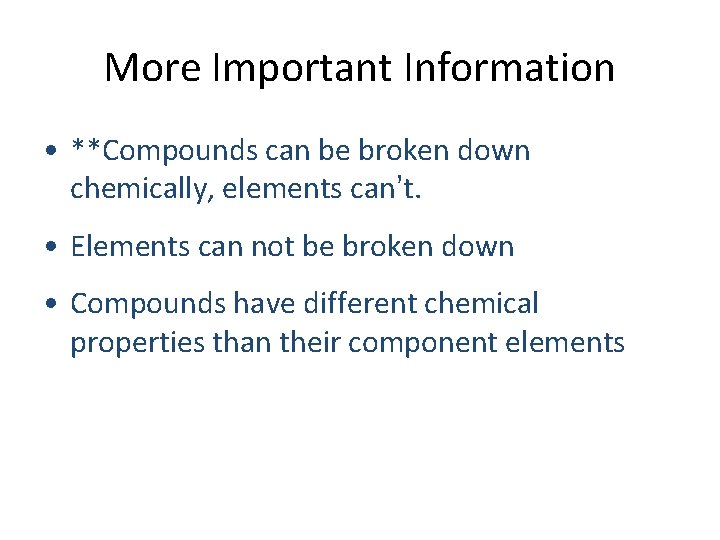
More Important Information • **Compounds can be broken down chemically, elements can’t. • Elements can not be broken down • Compounds have different chemical properties than their component elements

You Try… • Identify the following as an element or compound. WHY? • CH 4 • CO • K • Na. Cl. O • Cl • Ca. Cl 2
Elements of Life • Sulfur, Phosphorus, Oxygen, Nitrogen, Carbon, and Hydrogen are the “elements of life” • “SPONCH” • Living organisms are made of combinations of these elements
More Chemistry Periodic Table Basics
Subatomic Particles • Protons: positive, located in the nucleus, determines the atomic number (location on the periodic table) • Neutrons: neutral, located in the nucleus • Electrons: negative, located surrounding the nucleus, changes the charge of an atom (ion)
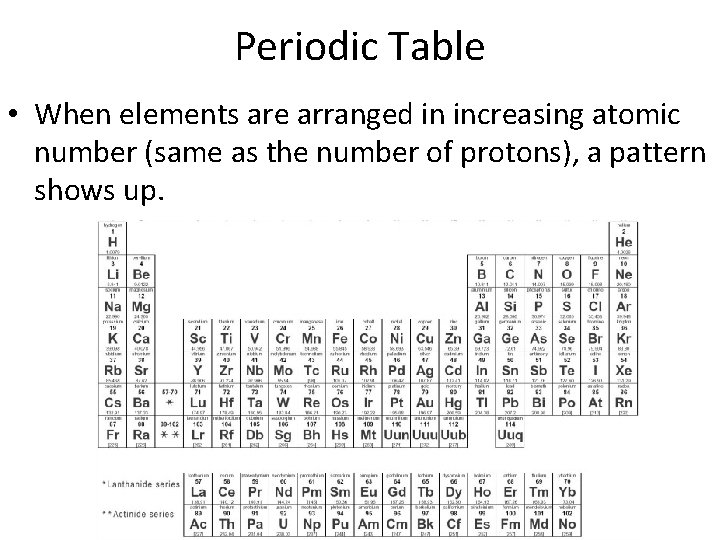
Periodic Table • When elements are arranged in increasing atomic number (same as the number of protons), a pattern shows up.
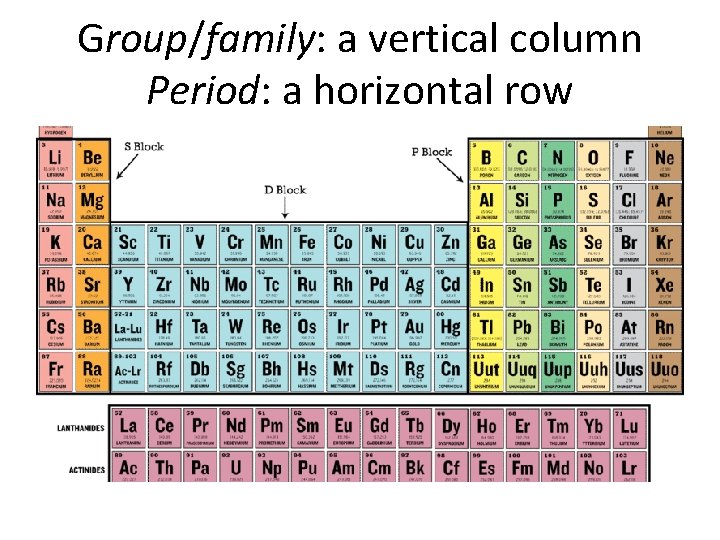
Group/family: a vertical column Period: a horizontal row
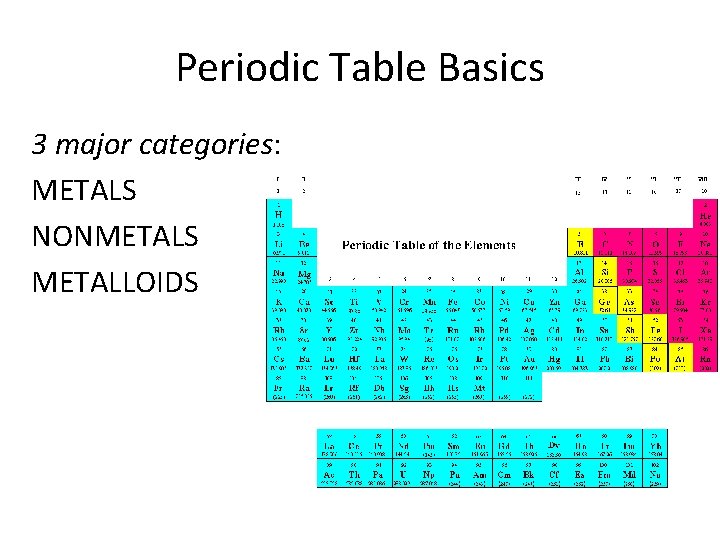
Periodic Table Basics 3 major categories: METALS NONMETALS METALLOIDS
- Slides: 11