Chemistry B 11 Chapter 5 Chemical Reactions Chemical

Chemistry B 11 Chapter 5 Chemical Reactions
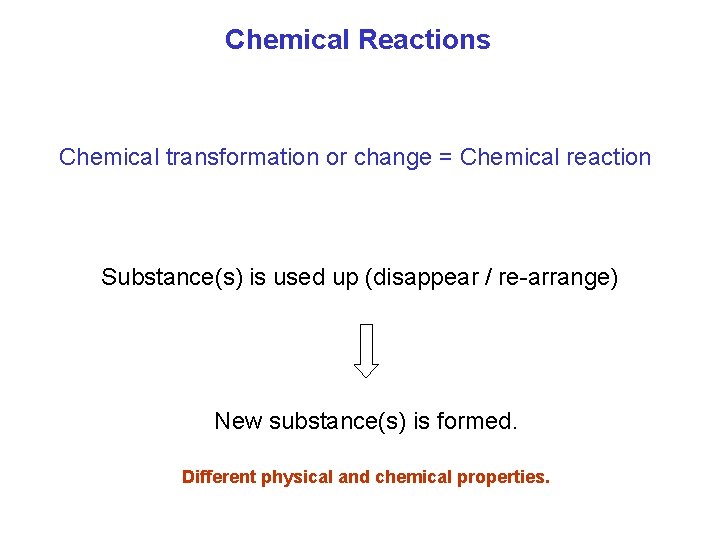
Chemical Reactions Chemical transformation or change = Chemical reaction Substance(s) is used up (disappear / re-arrange) New substance(s) is formed. Different physical and chemical properties.
Chemical Reactions
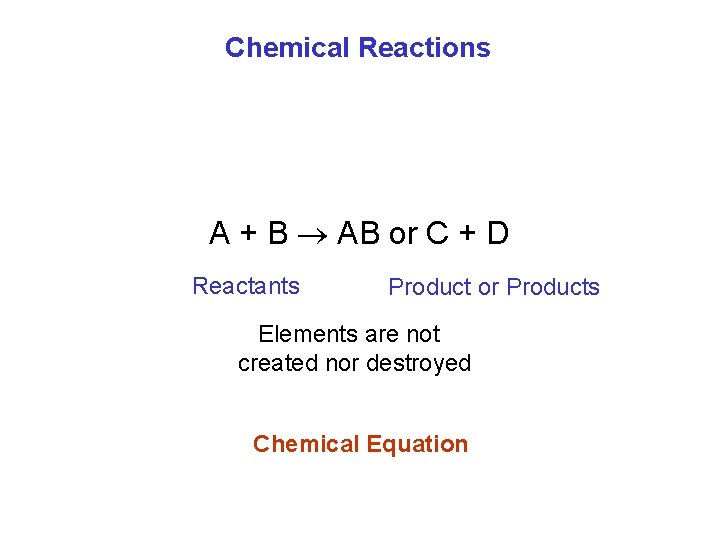
Chemical Reactions A + B AB or C + D Reactants Product or Products Elements are not created nor destroyed Chemical Equation
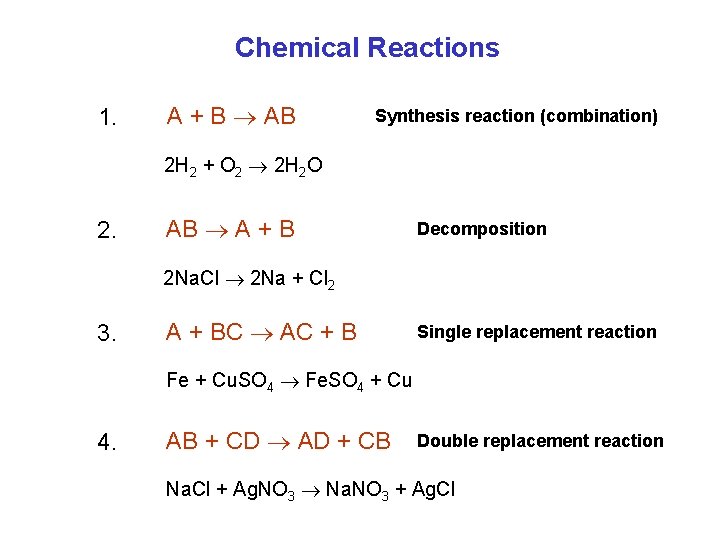
Chemical Reactions 1. A + B AB Synthesis reaction (combination) 2 H 2 + O 2 2 H 2 O 2. AB A + B Decomposition 2 Na. Cl 2 Na + Cl 2 3. A + BC AC + B Single replacement reaction Fe + Cu. SO 4 Fe. SO 4 + Cu 4. AB + CD AD + CB Double replacement reaction Na. Cl + Ag. NO 3 Na. NO 3 + Ag. Cl
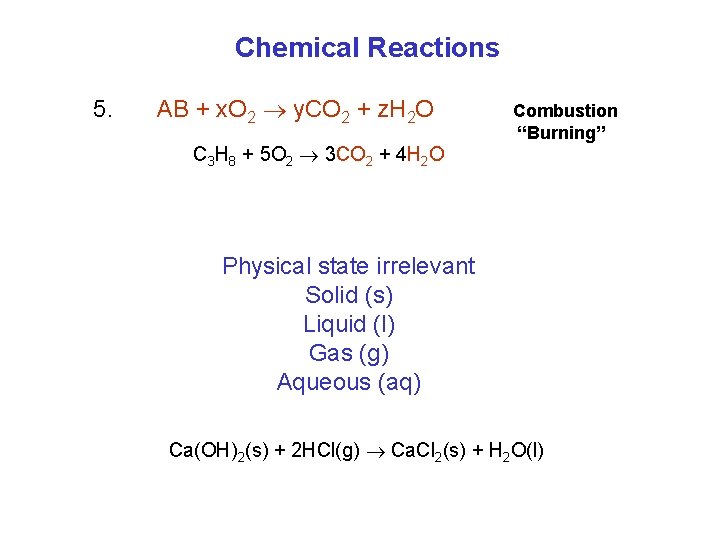
Chemical Reactions 5. AB + x. O 2 y. CO 2 + z. H 2 O C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O Combustion “Burning” Physical state irrelevant Solid (s) Liquid (l) Gas (g) Aqueous (aq) Ca(OH)2(s) + 2 HCl(g) Ca. Cl 2(s) + H 2 O(l)

Chemical reactions and heat Chemical reactions speed up With increased heat This is due to several reasons: 1) Activation energy 2) Increased energy 3) Quicker motion more interactions

Balance a chemical equation Why balancing?

Balance a chemical equation Law of conservation of mass Atoms are neither destroyed nor created. They shift from one substance to another. “Fruit Salad”
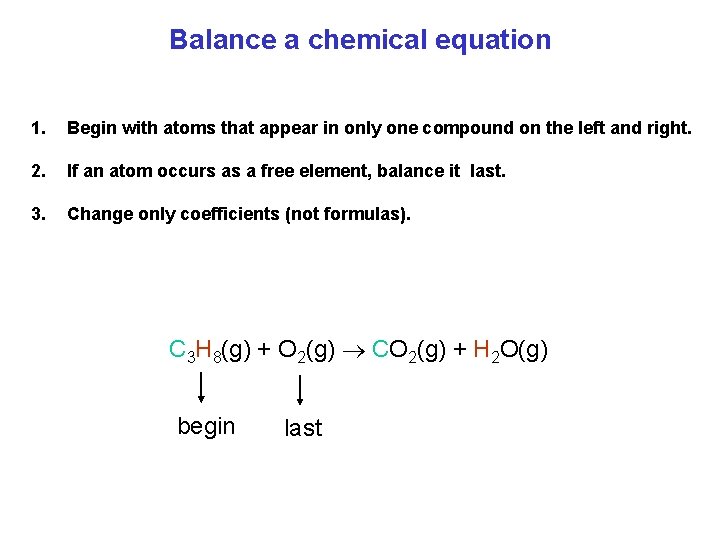
Balance a chemical equation 1. Begin with atoms that appear in only one compound on the left and right. 2. If an atom occurs as a free element, balance it last. 3. Change only coefficients (not formulas). C 3 H 8(g) + O 2(g) CO 2(g) + H 2 O(g) begin last
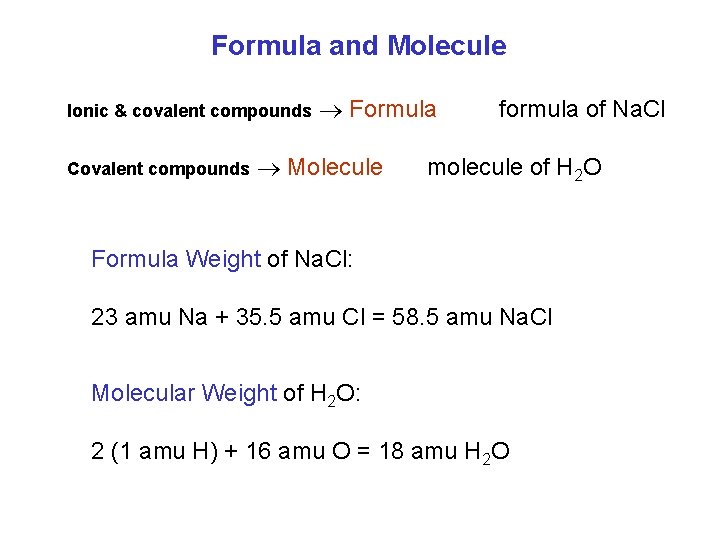
Formula and Molecule Ionic & covalent compounds Covalent compounds Formula Molecule formula of Na. Cl molecule of H 2 O Formula Weight of Na. Cl: 23 amu Na + 35. 5 amu Cl = 58. 5 amu Na. Cl Molecular Weight of H 2 O: 2 (1 amu H) + 16 amu O = 18 amu H 2 O
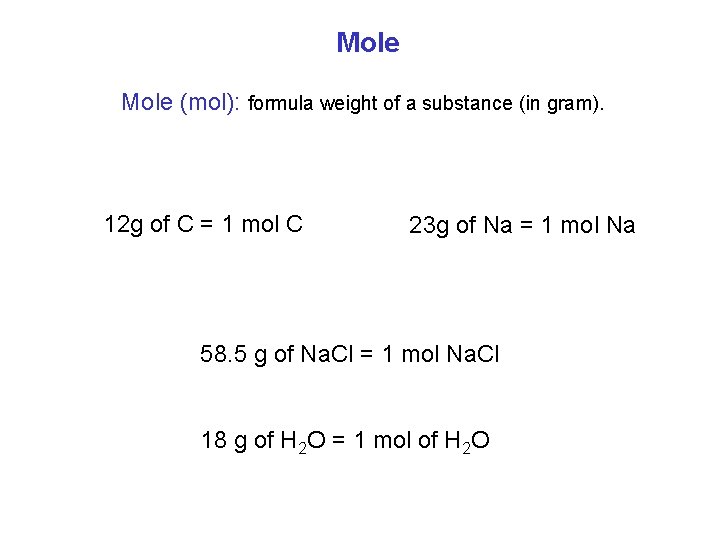
Mole (mol): formula weight of a substance (in gram). 12 g of C = 1 mol C 23 g of Na = 1 mol Na 58. 5 g of Na. Cl = 1 mol Na. Cl 18 g of H 2 O = 1 mol of H 2 O
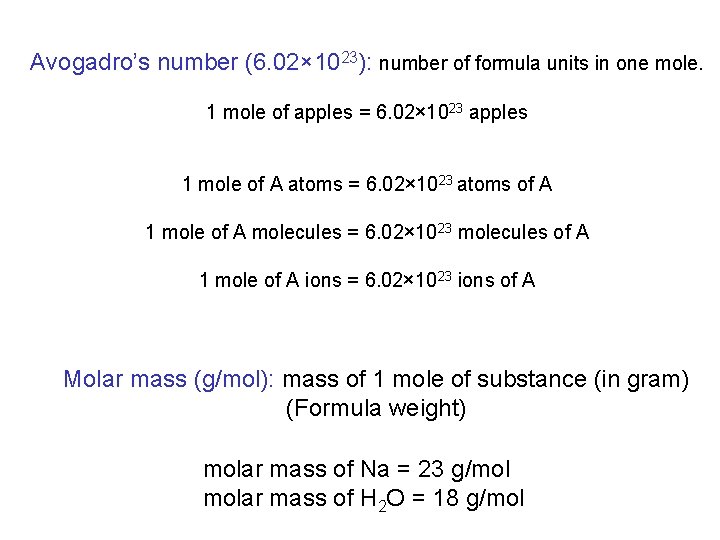
Avogadro’s number (6. 02× 1023): number of formula units in one mole. 1 mole of apples = 6. 02× 1023 apples 1 mole of A atoms = 6. 02× 1023 atoms of A 1 mole of A molecules = 6. 02× 1023 molecules of A 1 mole of A ions = 6. 02× 1023 ions of A Molar mass (g/mol): mass of 1 mole of substance (in gram) (Formula weight) molar mass of Na = 23 g/mol molar mass of H 2 O = 18 g/mol
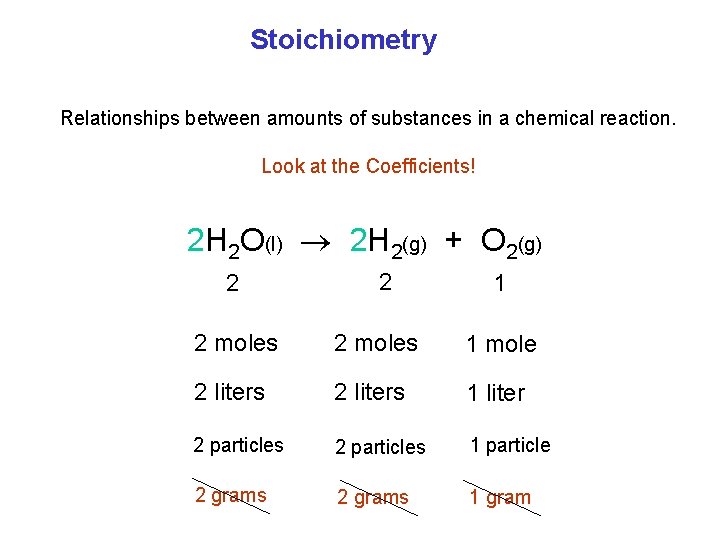
Stoichiometry Relationships between amounts of substances in a chemical reaction. Look at the Coefficients! 2 H 2 O(l) 2 H 2(g) + O 2(g) 2 2 1 2 moles 1 mole 2 liters 1 liter 2 particles 1 particle 2 grams 1 gram

mass volume A mole B mole Particle (atom) (molecule) (ion) mass 1 step: use coefficient in the balanced equation. CH 4 + 2 O 2 CO 2 + 2 H 2 O
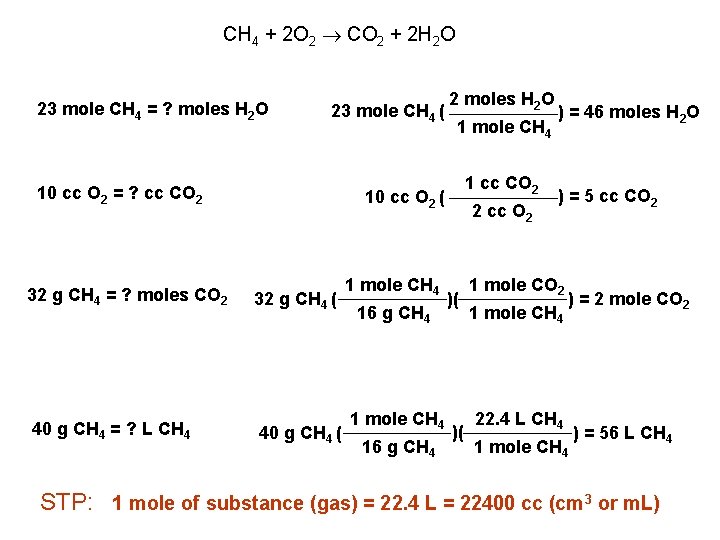
CH 4 + 2 O 2 CO 2 + 2 H 2 O 23 mole CH 4 = ? moles H 2 O 23 mole CH 4 ( 10 cc O 2 = ? cc CO 2 2 moles H 2 O 1 mole CH 4 1 cc CO 2 10 cc O 2 ( 32 g CH 4 = ? moles CO 2 32 g CH 4 ( 40 g CH 4 = ? L CH 4 40 g CH 4 ( 1 mole CH 4 16 g CH 4 2 cc O 2 )( )( ) = 46 moles H 2 O ) = 5 cc CO 2 1 mole CH 4 ) = 2 mole CO 2 22. 4 L CH 4 1 mole CH 4 ) = 56 L CH 4 STP: 1 mole of substance (gas) = 22. 4 L = 22400 cc (cm 3 or m. L)
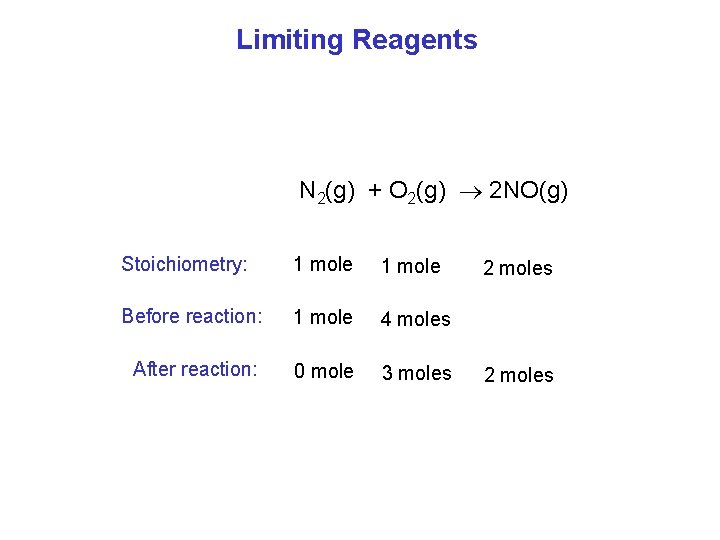
Limiting Reagents N 2(g) + O 2(g) 2 NO(g) Stoichiometry: 1 mole Before reaction: 1 mole 4 moles After reaction: 0 mole 3 moles 2 moles
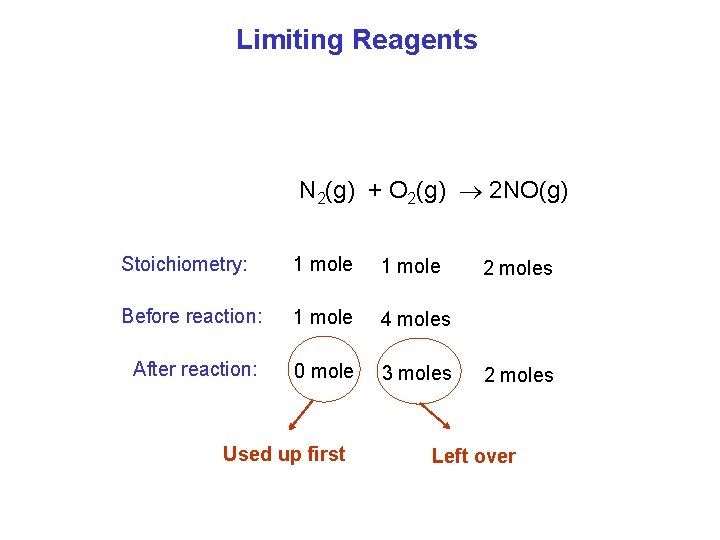
Limiting Reagents N 2(g) + O 2(g) 2 NO(g) Stoichiometry: 1 mole Before reaction: 1 mole 4 moles After reaction: 0 mole 3 moles Used up first 2 moles Left over
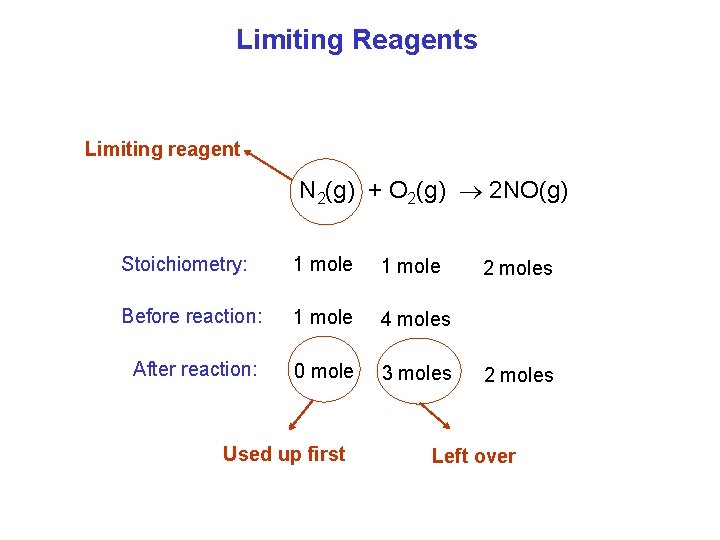
Limiting Reagents Limiting reagent N 2(g) + O 2(g) 2 NO(g) Stoichiometry: 1 mole Before reaction: 1 mole 4 moles After reaction: 0 mole 3 moles Used up first 2 moles Left over
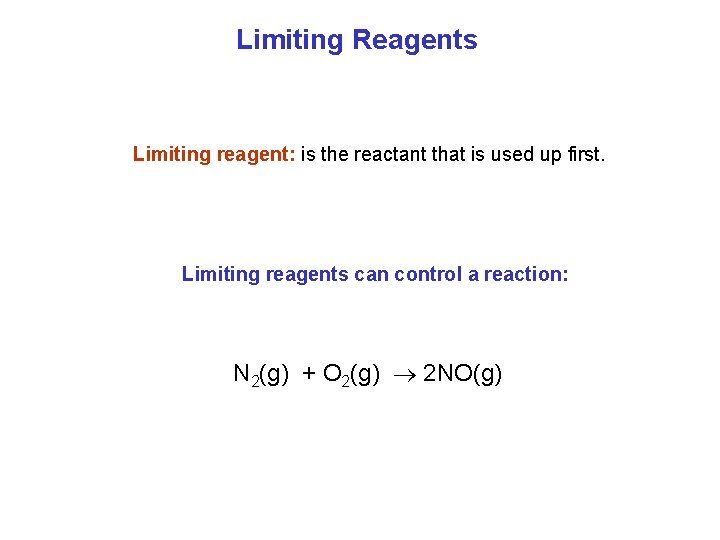
Limiting Reagents Limiting reagent: is the reactant that is used up first. Limiting reagents can control a reaction: N 2(g) + O 2(g) 2 NO(g)
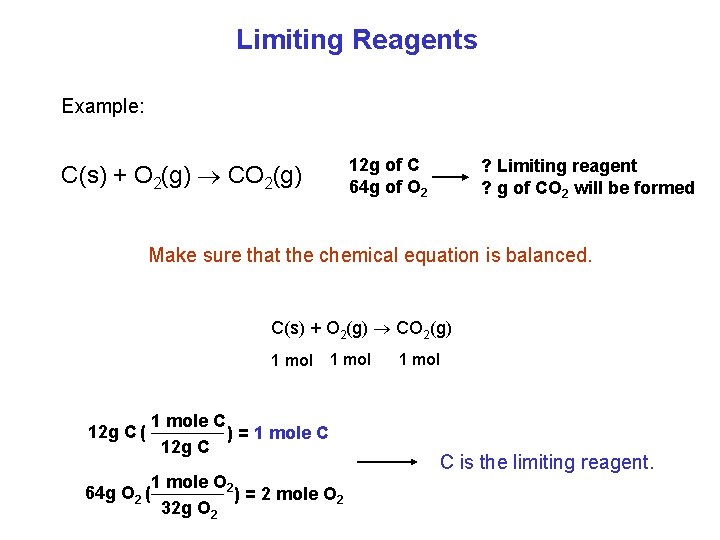
Limiting Reagents Example: 12 g of C 64 g of O 2 C(s) + O 2(g) CO 2(g) ? Limiting reagent ? g of CO 2 will be formed Make sure that the chemical equation is balanced. C(s) + O 2(g) CO 2(g) 1 mol 12 g C ( 64 g O 2 ( 1 mole C ) = 1 mole C 12 g C 1 mole O 2 ) = 2 mole O 2 32 g O 2 1 mol C is the limiting reagent.
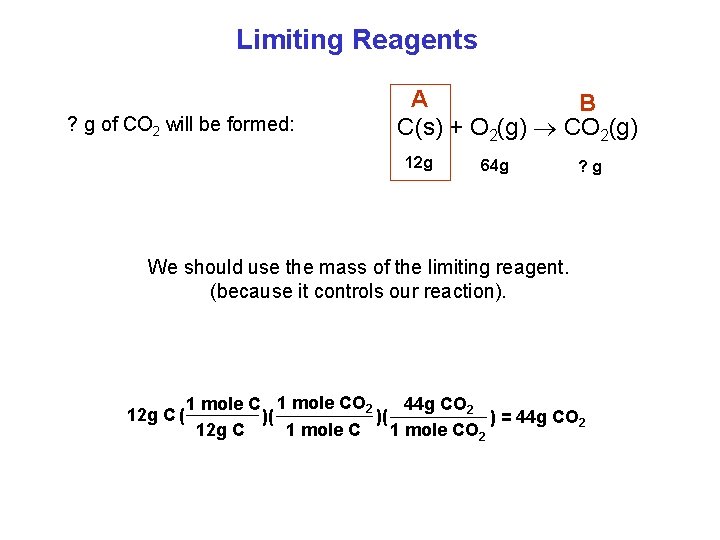
Limiting Reagents ? g of CO 2 will be formed: A B C(s) + O 2(g) CO 2(g) 12 g 64 g ? g We should use the mass of the limiting reagent. (because it controls our reaction). 12 g C ( 1 mole CO 2 44 g CO 2 )( )( ) = 44 g CO 2 12 g C 1 mole CO 2
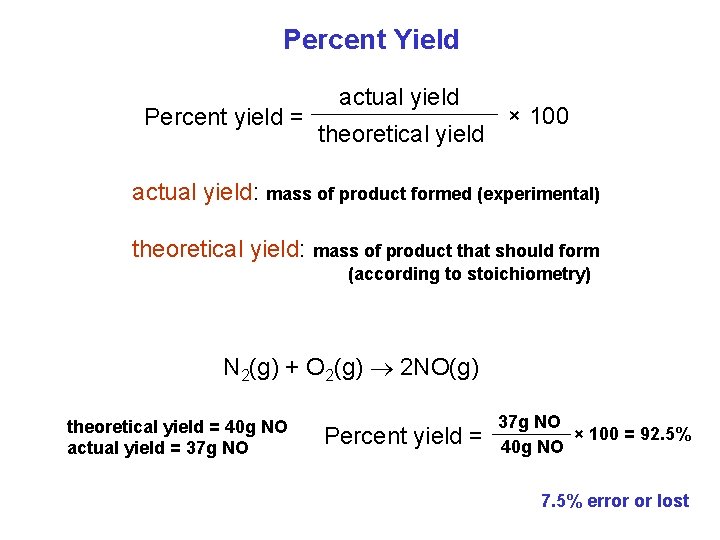
Percent Yield Percent yield = actual yield theoretical yield × 100 actual yield: mass of product formed (experimental) theoretical yield: mass of product that should form (according to stoichiometry) N 2(g) + O 2(g) 2 NO(g) theoretical yield = 40 g NO actual yield = 37 g NO Percent yield = 37 g NO × 100 = 92. 5% 40 g NO 7. 5% error or lost
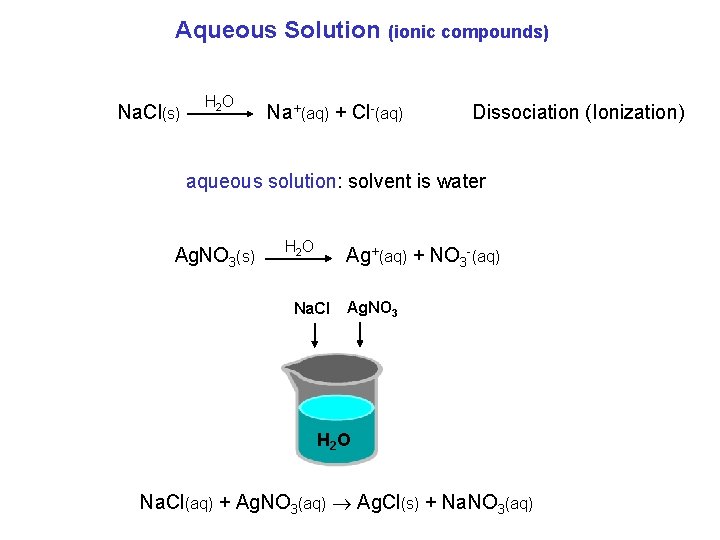
Aqueous Solution Na. Cl(s) H 2 O (ionic compounds) Na+(aq) + Cl-(aq) Dissociation (Ionization) aqueous solution: solvent is water Ag. NO 3(s) H 2 O Ag+(aq) + NO 3 -(aq) Na. Cl Ag. NO 3 H 2 O Na. Cl(aq) + Ag. NO 3(aq) Ag. Cl(s) + Na. NO 3(aq)
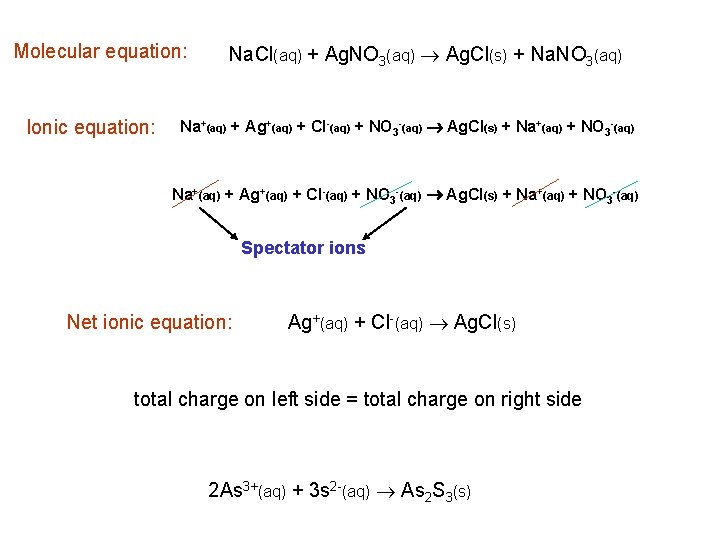
Molecular equation: Ionic equation: Na. Cl(aq) + Ag. NO 3(aq) Ag. Cl(s) + Na. NO 3(aq) Na+(aq) + Ag+(aq) + Cl-(aq) + NO 3 -(aq) Ag. Cl(s) + Na+(aq) + NO 3 -(aq) Spectator ions Net ionic equation: Ag+(aq) + Cl-(aq) Ag. Cl(s) total charge on left side = total charge on right side 2 As 3+(aq) + 3 s 2 -(aq) As 2 S 3(s)
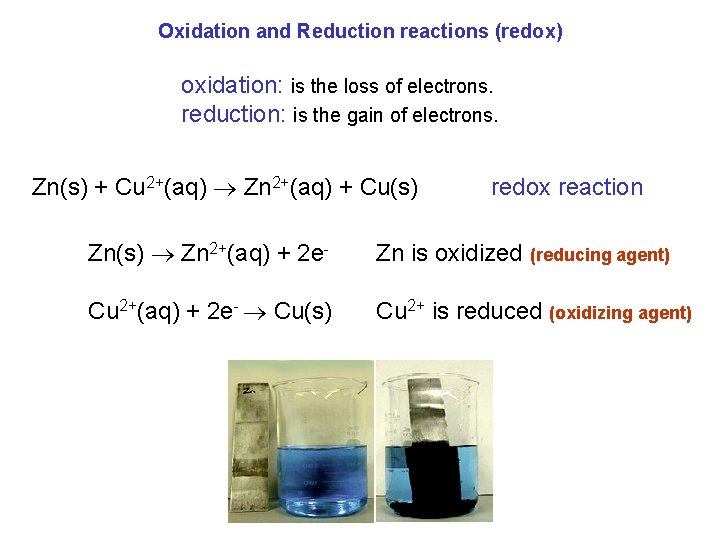
Oxidation and Reduction reactions (redox) oxidation: is the loss of electrons. reduction: is the gain of electrons. Zn(s) + Cu 2+(aq) Zn 2+(aq) + Cu(s) redox reaction Zn(s) Zn 2+(aq) + 2 e- Zn is oxidized (reducing agent) Cu 2+(aq) + 2 e- Cu(s) Cu 2+ is reduced (oxidizing agent)
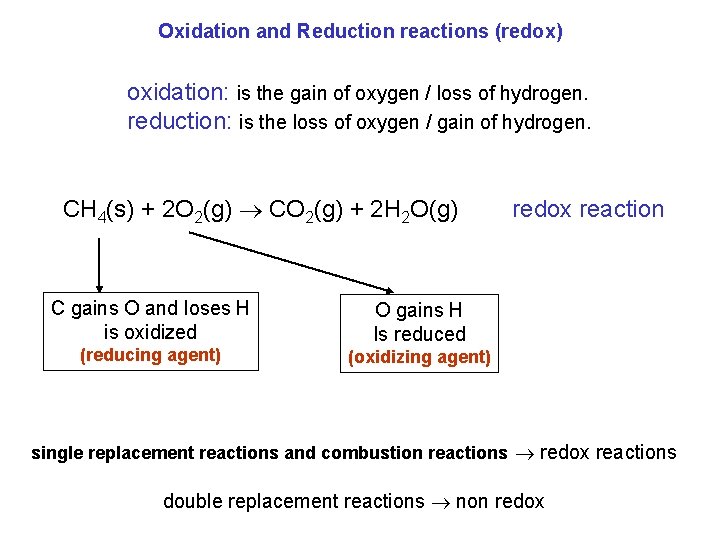
Oxidation and Reduction reactions (redox) oxidation: is the gain of oxygen / loss of hydrogen. reduction: is the loss of oxygen / gain of hydrogen. CH 4(s) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) C gains O and loses H is oxidized (reducing agent) redox reaction O gains H Is reduced (oxidizing agent) single replacement reactions and combustion reactions redox reactions double replacement reactions non redox
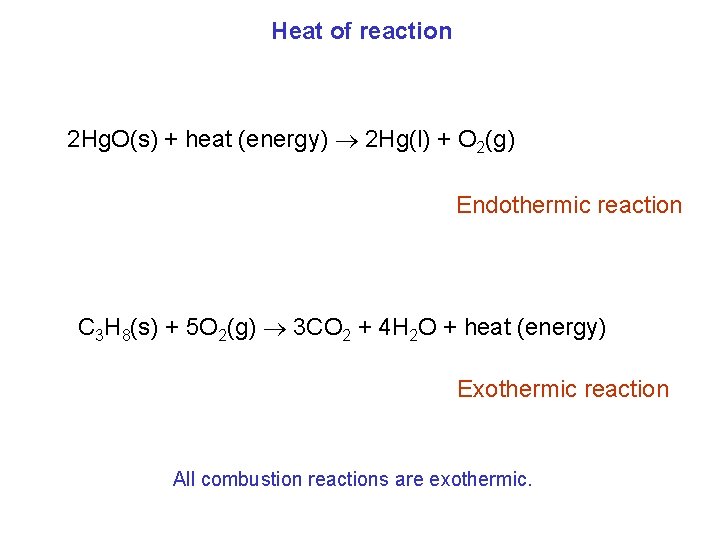
Heat of reaction 2 Hg. O(s) + heat (energy) 2 Hg(l) + O 2(g) Endothermic reaction C 3 H 8(s) + 5 O 2(g) 3 CO 2 + 4 H 2 O + heat (energy) Exothermic reaction All combustion reactions are exothermic.
- Slides: 28