Chemistry B 11 Chapter 15 Lipids Lipids Family

Chemistry B 11 Chapter 15 Lipids

Lipids - Family of biomolecules. - They are not defined by a particular functional group, thus they have a variety of structures and functions. - They are soluble in organic solvents but not in water (nonpolar). - They contain many nonpolar C—C and C—H bonds and few polar bonds resulting in their water insolubility.
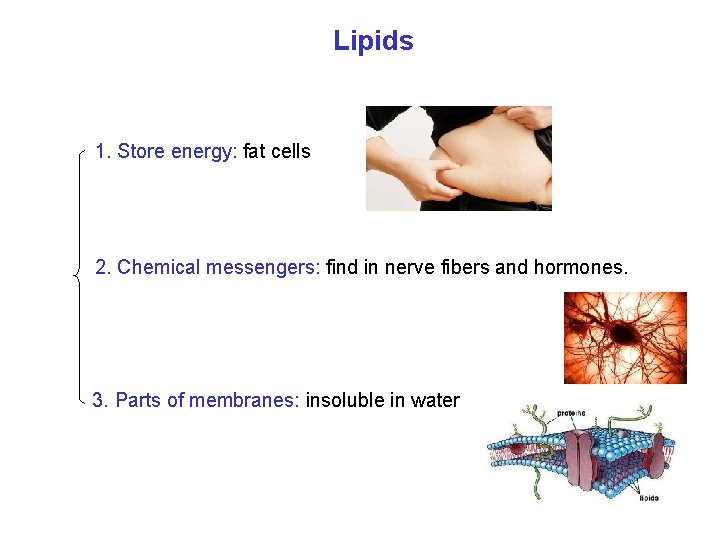
Lipids 1. Store energy: fat cells 2. Chemical messengers: find in nerve fibers and hormones. 3. Parts of membranes: insoluble in water
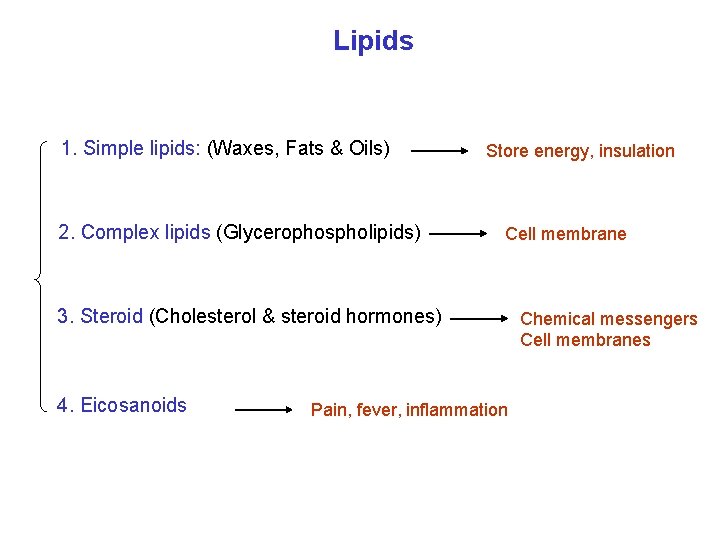
Lipids 1. Simple lipids: (Waxes, Fats & Oils) 2. Complex lipids (Glycerophospholipids) Store energy, insulation Cell membrane 3. Steroid (Cholesterol & steroid hormones) 4. Eicosanoids Pain, fever, inflammation Chemical messengers Cell membranes
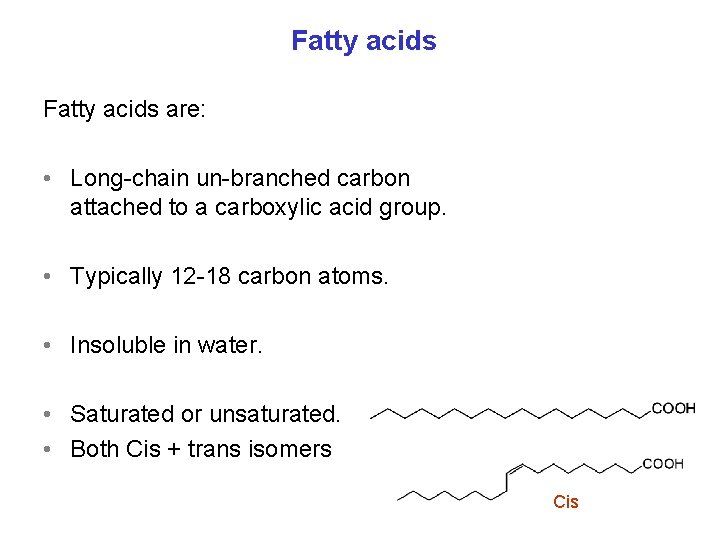
Fatty acids are: • Long-chain un-branched carbon attached to a carboxylic acid group. • Typically 12 -18 carbon atoms. • Insoluble in water. • Saturated or unsaturated. • Both Cis + trans isomers Cis
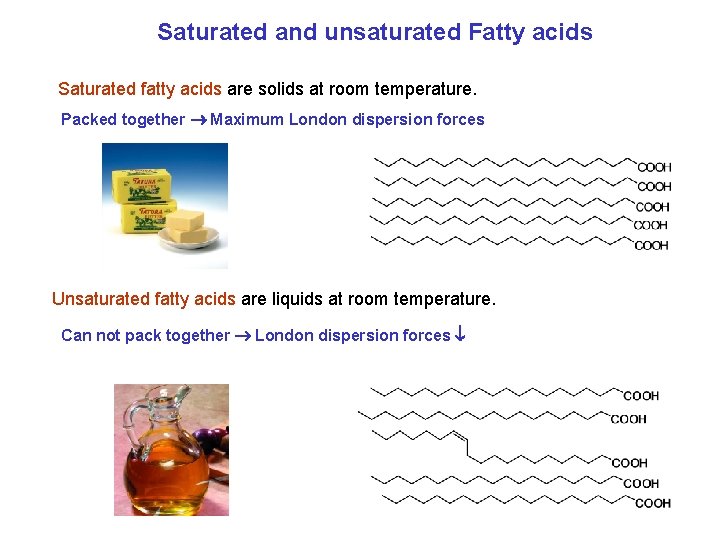
Saturated and unsaturated Fatty acids Saturated fatty acids are solids at room temperature. Packed together Maximum London dispersion forces Unsaturated fatty acids are liquids at room temperature. Can not pack together London dispersion forces

Fatty acids • The human body is capable of synthesizing most fatty acids from carbohydrates or other fatty acids. • Humans do not synthesize sufficient amounts of fatty acids that have more than one double bond. • More than one double bond fatty acids are called essential fatty acids and they must be provided in the diet.

Waxes - are found in many plants and animals (or humans). - In plants, they help prevent loss of water and damage from pests. - In humans and animals, provide waterproof coating on skin and fur. Wax is an ester of saturated fatty acid and long chain alcohol. Long-chain alcohol Ester bond Fatty acid
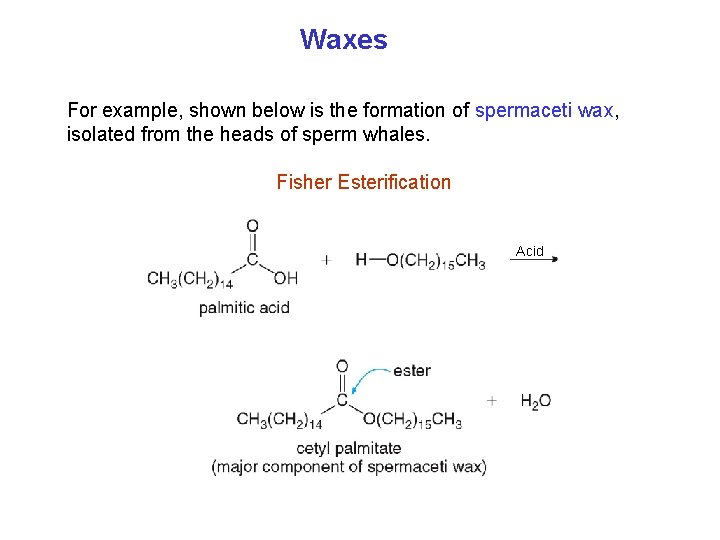
Waxes For example, shown below is the formation of spermaceti wax, isolated from the heads of sperm whales. Fisher Esterification Acid
Beeswax Carnauba Coating Jojoba Lanolin from wool lotions

Triacylglycerols (Triglycerides) Triacylglycerols are: • Fats and oils (are stored in the body). • Triesters of glycerol. • Produced by Fischer esterification. • Formed when the hydroxyl groups of glycerol react with the carboxyl groups of fatty acids.
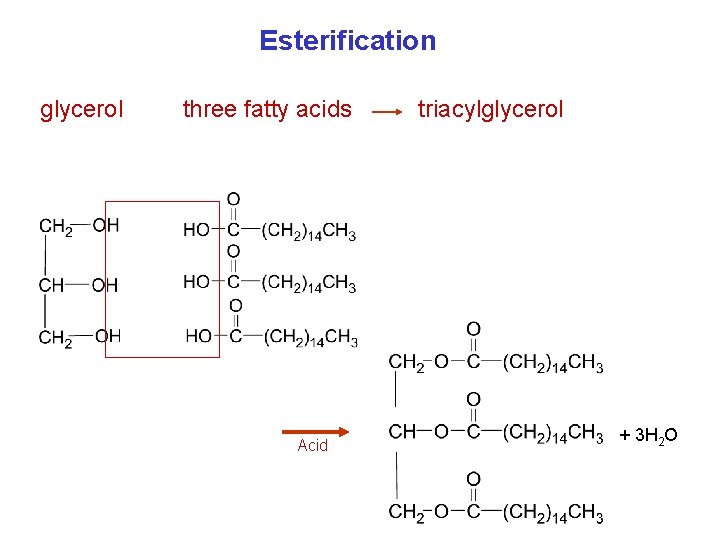
Esterification glycerol three fatty acids Acid triacylglycerol + 3 H 2 O
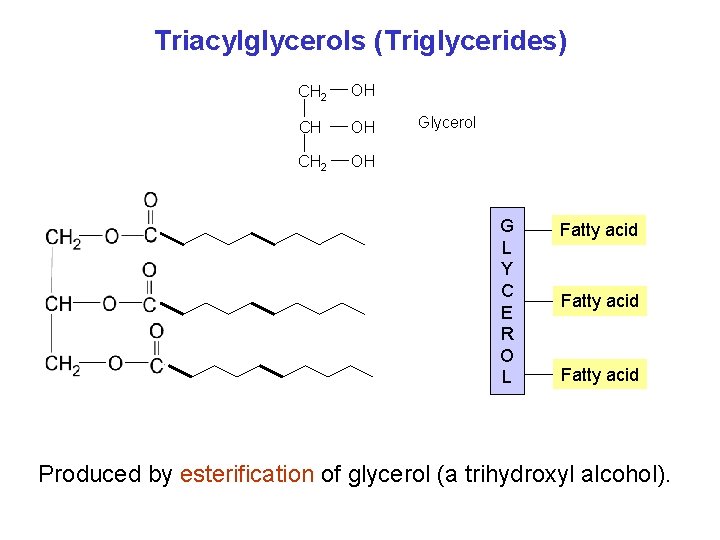
Triacylglycerols (Triglycerides) CH 2 OH Glycerol G L Y C E R O L Fatty acid Produced by esterification of glycerol (a trihydroxyl alcohol).

Triacylglycerols (Triglycerides) Fat: is a triacylglycerol that is solid at room temperature. Made by more saturated fatty acids (Saturated triacylglycerols). Meat, milk, butter and cheese (animal sources). Oil: is a triacylglycerol that is liquid at room temperature. Made by more unsaturated fatty acids (Unsaturated triacylglycerols). Corn, cotton seed, safflower and sunflower (plant sources). Both are colorless, odorless, and tasteless.
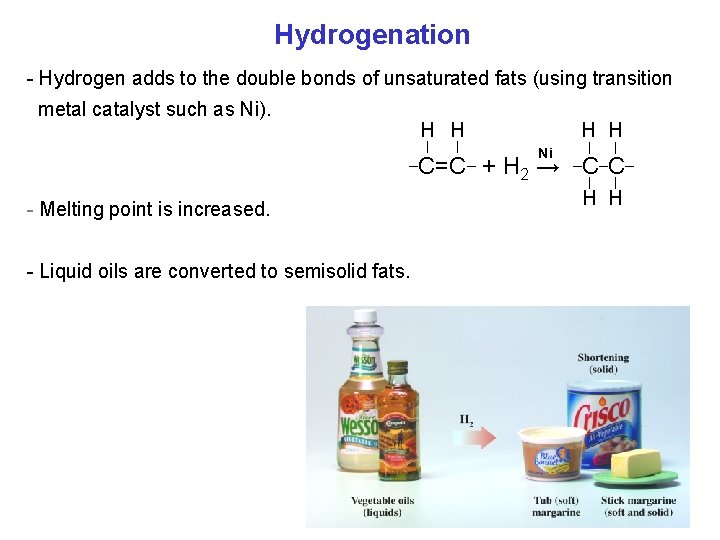
Hydrogenation - Hydrogen adds to the double bonds of unsaturated fats (using transition metal catalyst such as Ni). H H _ - Melting point is increased. - Liquid oils are converted to semisolid fats. H H _ Ni C=C + H 2 → _C_C_ H H

1 - Hydrogenation Ni + 3 H 2 glyceryl Trioleate (triolein) glyceryl tristearate (tristearin)
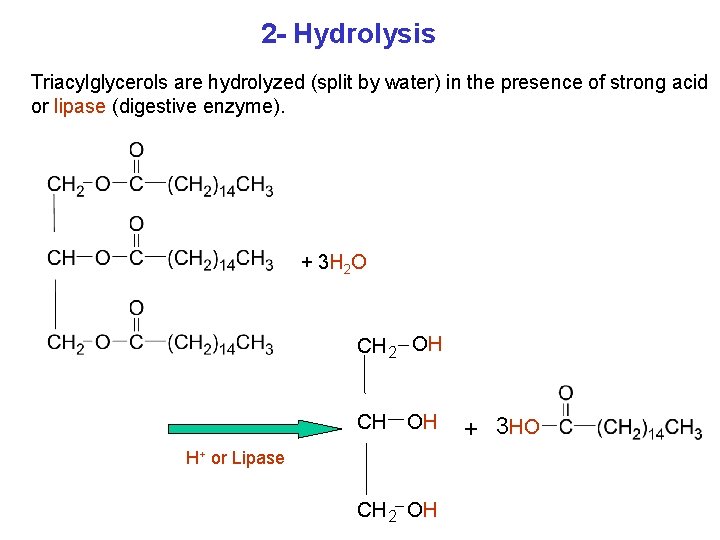
2 - Hydrolysis Triacylglycerols are hydrolyzed (split by water) in the presence of strong acid or lipase (digestive enzyme). + 3 H 2 O CH 2 OH CH OH H+ or Lipase CH 2 OH + 3 HO

3 - Saponification • Is the process of forming “soaps” (salts of fatty acids). • Is the reaction of a fat with a strong base (Na. OH). • Splits triacylglycerols into glycerol and the salts of fatty acids. • With KOH or the oils that are polyunsaturated gives softer soaps (liquid soaps). • Name of soap gives the source of the oil. Like coconut or avocado soap
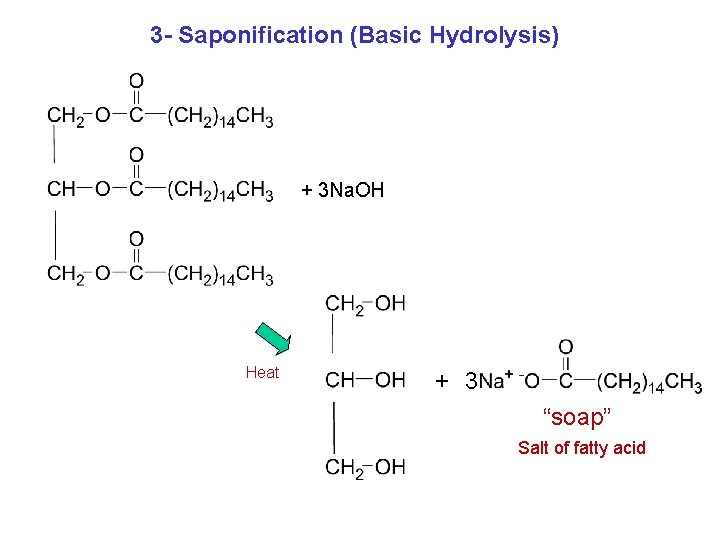
3 - Saponification (Basic Hydrolysis) + 3 Na. OH Heat + 3 “soap” Salt of fatty acid
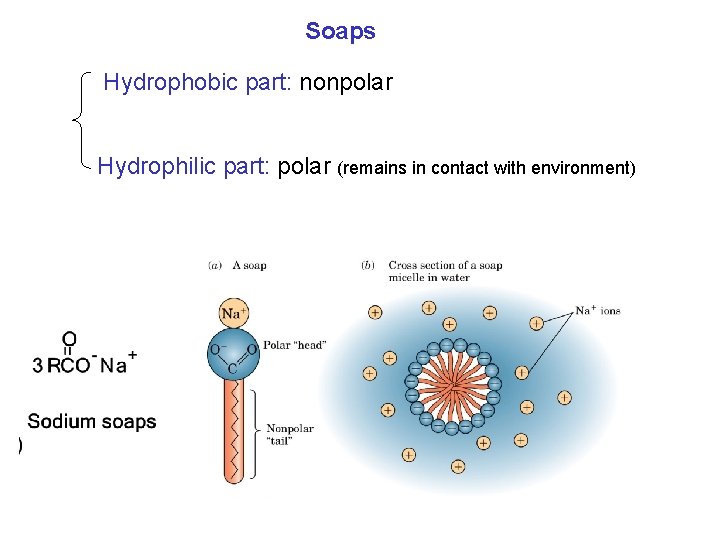
Soaps Hydrophobic part: nonpolar Hydrophilic part: polar (remains in contact with environment)
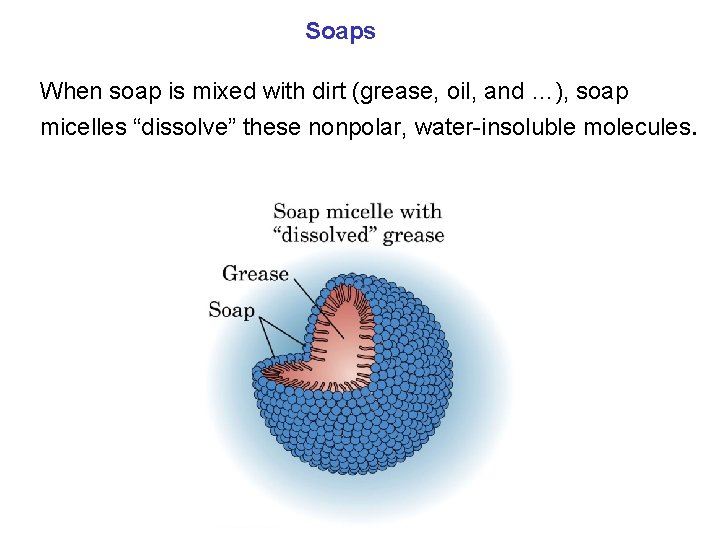
Soaps When soap is mixed with dirt (grease, oil, and …), soap micelles “dissolve” these nonpolar, water-insoluble molecules.
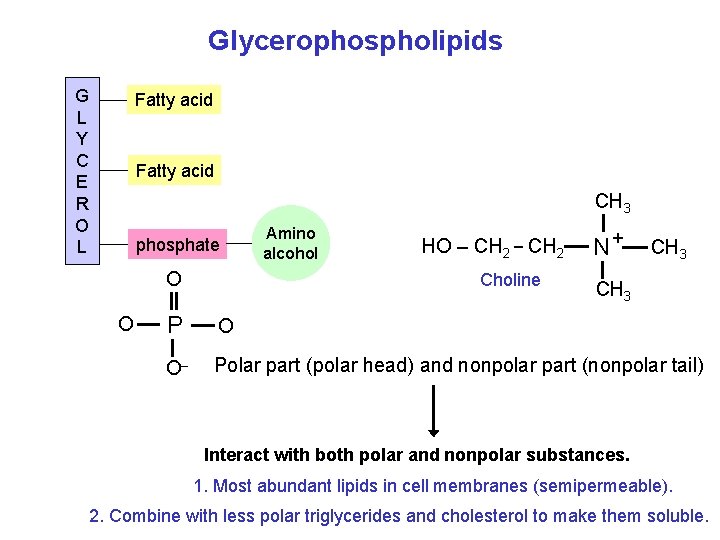
Glycerophospholipids G L Y C E R O L Fatty acid CH 3 phosphate O O P O _ Amino alcohol HO – CH 2 _ CH 2 Choline N+ CH 3 O Polar part (polar head) and nonpolar part (nonpolar tail) Interact with both polar and nonpolar substances. 1. Most abundant lipids in cell membranes (semipermeable). 2. Combine with less polar triglycerides and cholesterol to make them soluble.
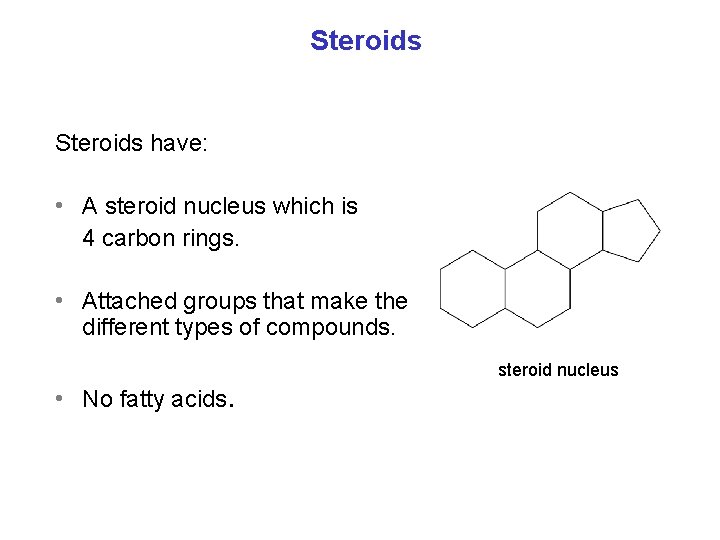
Steroids have: • A steroid nucleus which is 4 carbon rings. • Attached groups that make the different types of compounds. • No fatty acids. steroid nucleus
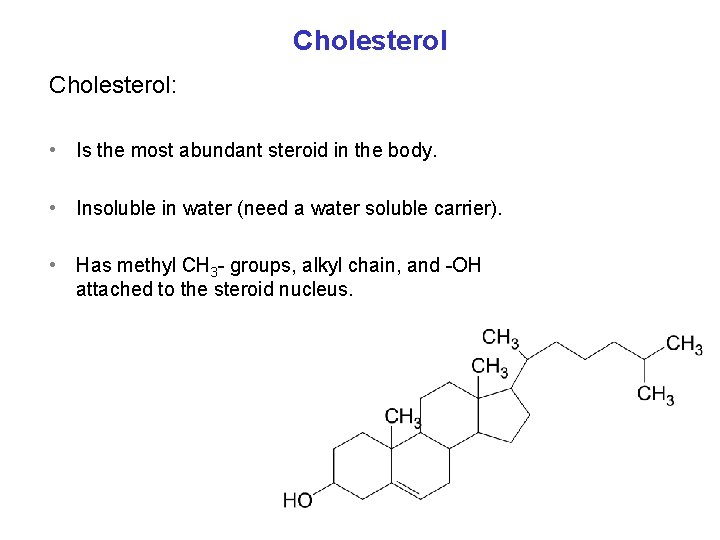
Cholesterol: • Is the most abundant steroid in the body. • Insoluble in water (need a water soluble carrier). • Has methyl CH 3 - groups, alkyl chain, and -OH attached to the steroid nucleus.
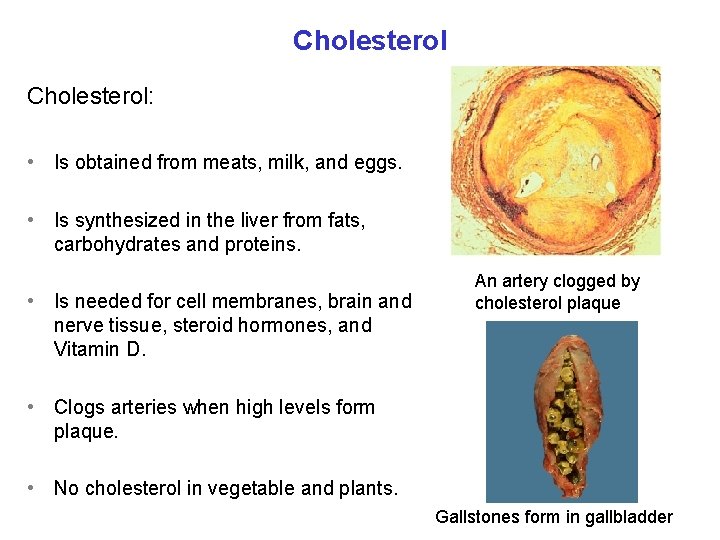
Cholesterol: • Is obtained from meats, milk, and eggs. • Is synthesized in the liver from fats, carbohydrates and proteins. • Is needed for cell membranes, brain and nerve tissue, steroid hormones, and Vitamin D. An artery clogged by cholesterol plaque • Clogs arteries when high levels form plaque. • No cholesterol in vegetable and plants. Gallstones form in gallbladder
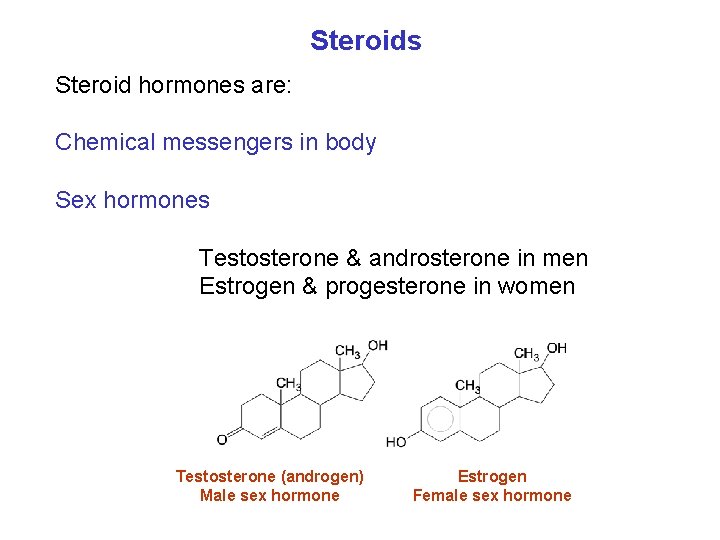
Steroids Steroid hormones are: Chemical messengers in body Sex hormones Testosterone & androsterone in men Estrogen & progesterone in women Testosterone (androgen) Male sex hormone Estrogen Female sex hormone
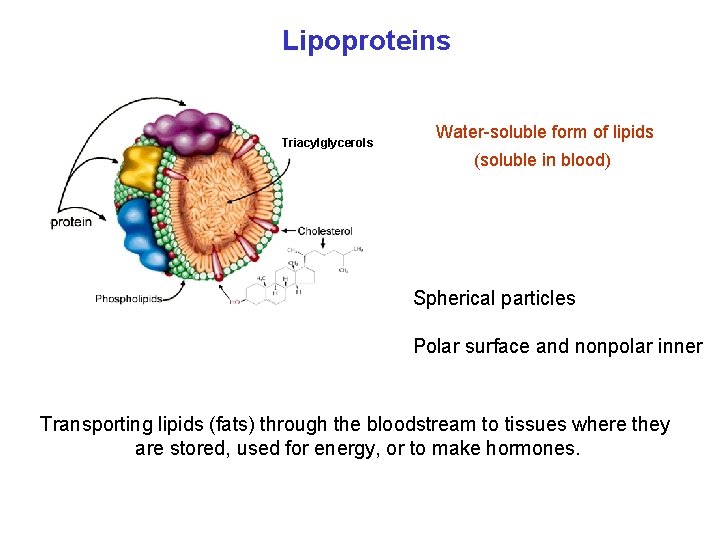
Lipoproteins Triacylglycerols Water-soluble form of lipids (soluble in blood) Spherical particles Polar surface and nonpolar inner Transporting lipids (fats) through the bloodstream to tissues where they are stored, used for energy, or to make hormones.
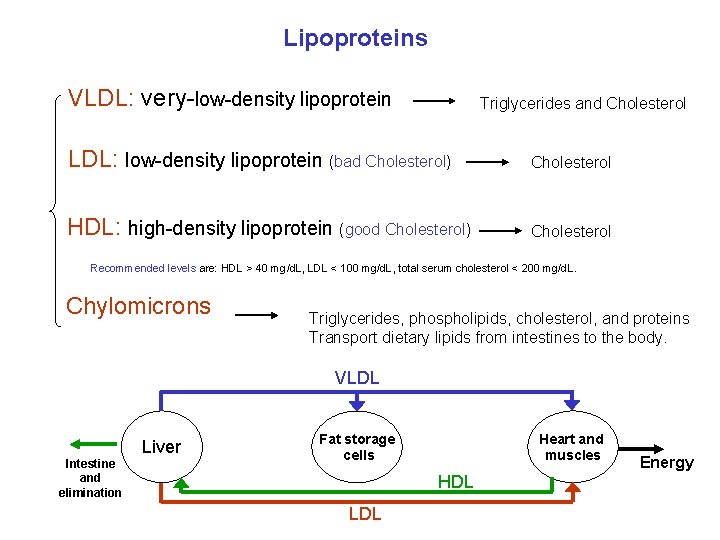
Lipoproteins VLDL: very-low-density lipoprotein Triglycerides and Cholesterol LDL: low-density lipoprotein (bad Cholesterol) Cholesterol HDL: high-density lipoprotein (good Cholesterol) Cholesterol Recommended levels are: HDL > 40 mg/d. L, LDL < 100 mg/d. L, total serum cholesterol < 200 mg/d. L. Chylomicrons Triglycerides, phospholipids, cholesterol, and proteins Transport dietary lipids from intestines to the body. VLDL Intestine and elimination Liver Heart and muscles Fat storage cells HDL LDL Energy
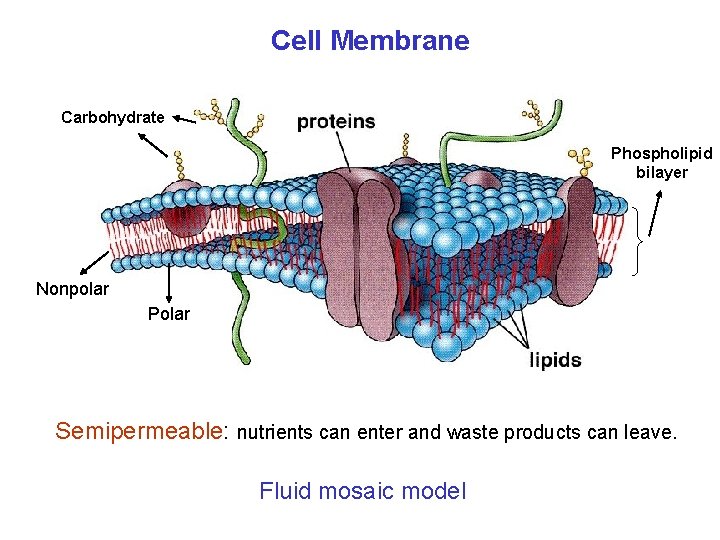
Cell Membrane Carbohydrate Phospholipid bilayer Nonpolar Polar Semipermeable: nutrients can enter and waste products can leave. Fluid mosaic model
- Slides: 29