Chemistry AP Biology Chemical Bonds Electronegativity how well
Chemistry AP Biology
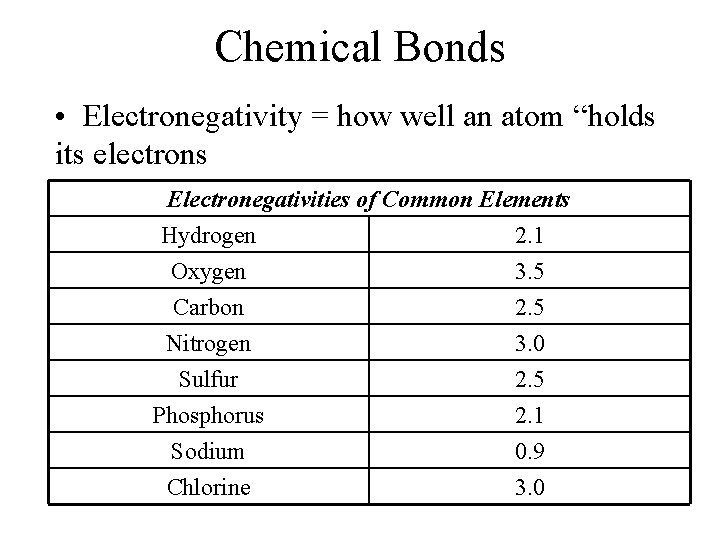
Chemical Bonds • Electronegativity = how well an atom “holds its electrons Electronegativities of Common Elements Hydrogen 2. 1 Oxygen 3. 5 Carbon 2. 5 Nitrogen Sulfur Phosphorus Sodium Chlorine 3. 0 2. 5 2. 1 0. 9 3. 0
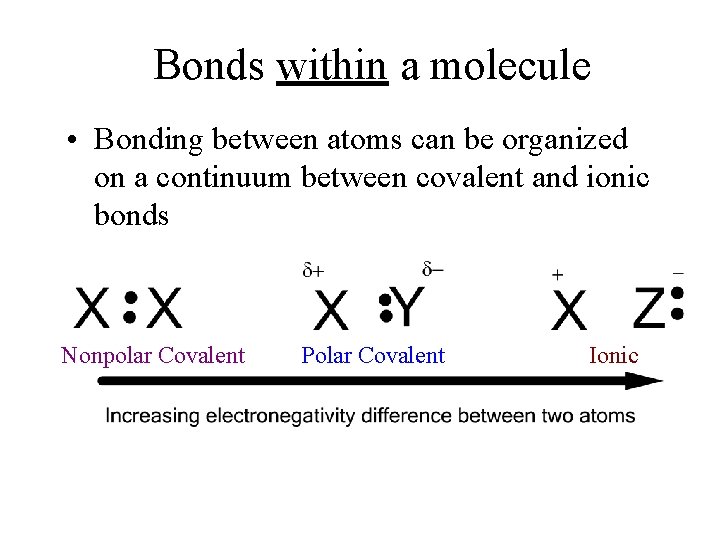
Bonds within a molecule • Bonding between atoms can be organized on a continuum between covalent and ionic bonds Nonpolar Covalent Polar Covalent Ionic
Bonds within a molecule • Nonpolar covalent bond = electrons are shared evenly between atoms • Polar covalent bond = electrons are shared but are more tightly held by one atom • Ionic bond = Electrons are not shared due to strong electronegativity differences (one atom “grabs” the electrons from the other)
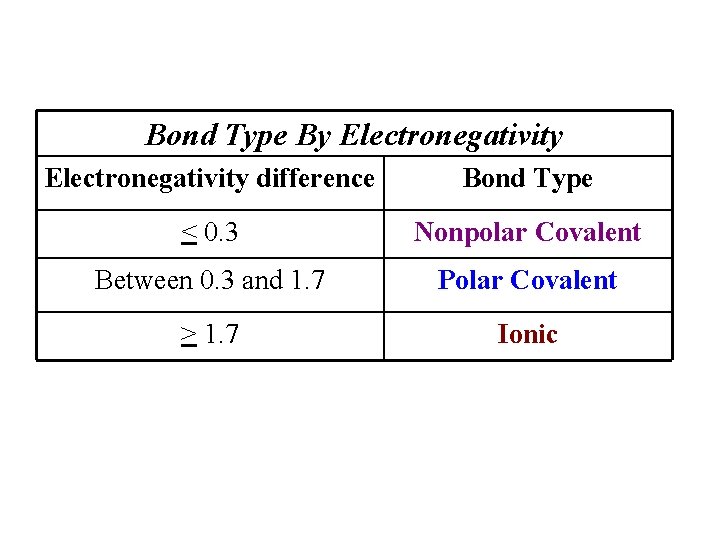
Bond Type By Electronegativity difference Bond Type < 0. 3 Nonpolar Covalent Between 0. 3 and 1. 7 Polar Covalent > 1. 7 Ionic
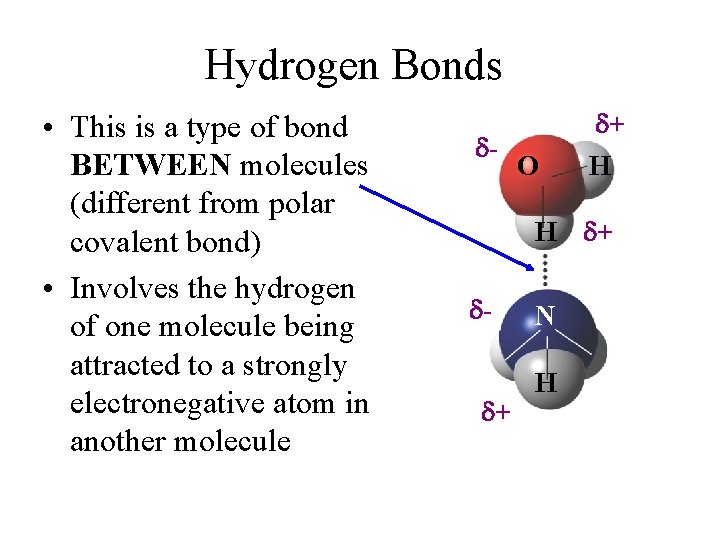
Hydrogen Bonds • This is a type of bond BETWEEN molecules (different from polar covalent bond) • Involves the hydrogen of one molecule being attracted to a strongly electronegative atom in another molecule d- O d+ H H d+ dd+ N H
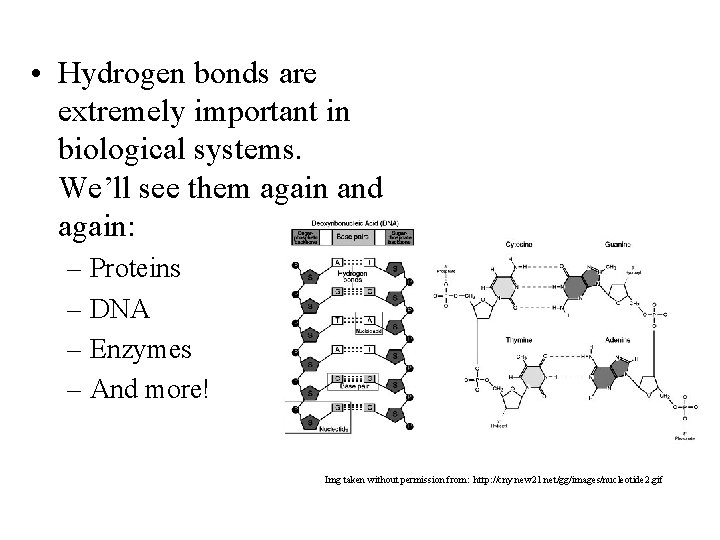
• Hydrogen bonds are extremely important in biological systems. We’ll see them again and again: – Proteins – DNA – Enzymes – And more! Img taken without permission from: http: //cny. new 21. net/gg/images/nucleotide 2. gif

Van der Waals Interactions • Weak attractions when two molecules are very close together • Temporary dipole moments in an atom can cause these tiny attractions Images taken without permission from: http: //news. bbc. co. uk/1/hi/sci/tech/781611. stm

Applications… • Stickybot– uses same model as gecko feet! • Gecko glue Image taken without permission from http: //www. sciencedaily. com/releases/2010/08/100826104135. htm

Properties of Water • Polar molecule forms hydrogen bonds • Hydrogen bonds give water some special characteristics (not all): – High specific heat – Evaporative cooling – Insulation of bodies of water
Properties of water (cont. ) • High Specific Heat – Takes a lot of energy to heat up 1 degree – Makes water very stable • Evaporative cooling – Water has a high heat of vaporization – Evaporative cooling prevents overheating
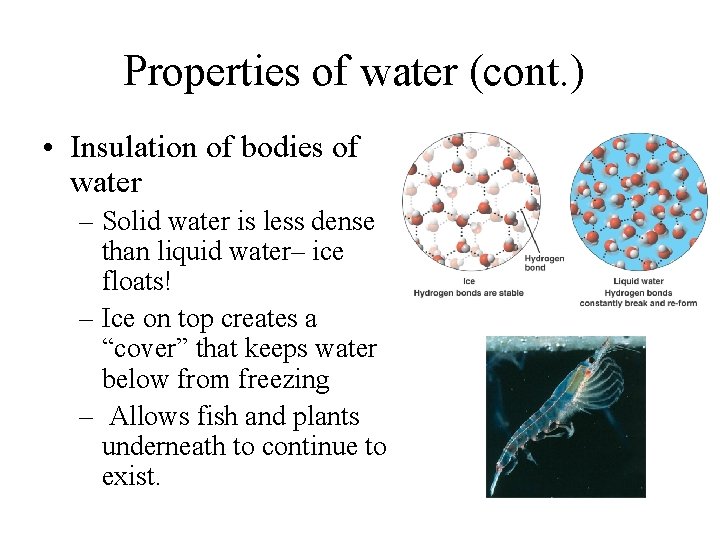
Properties of water (cont. ) • Insulation of bodies of water – Solid water is less dense than liquid water– ice floats! – Ice on top creates a “cover” that keeps water below from freezing – Allows fish and plants underneath to continue to exist.
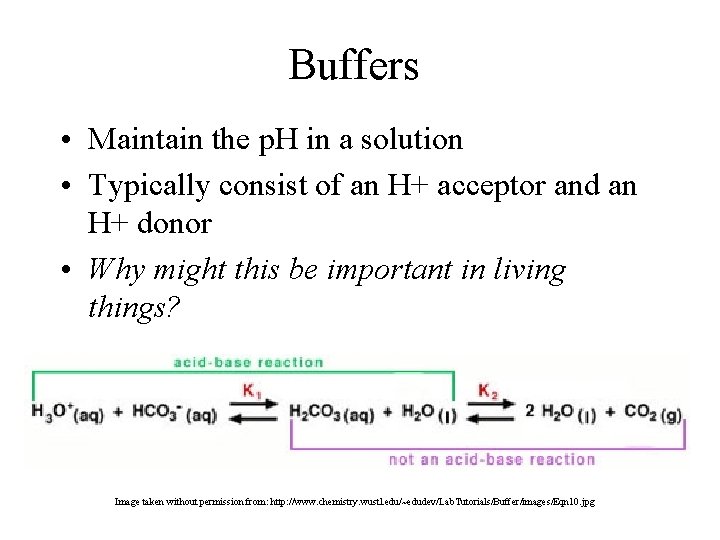
Buffers • Maintain the p. H in a solution • Typically consist of an H+ acceptor and an H+ donor • Why might this be important in living things? Image taken without permission from: http: //www. chemistry. wustl. edu/~edudev/Lab. Tutorials/Buffer/images/Eqn 10. jpg
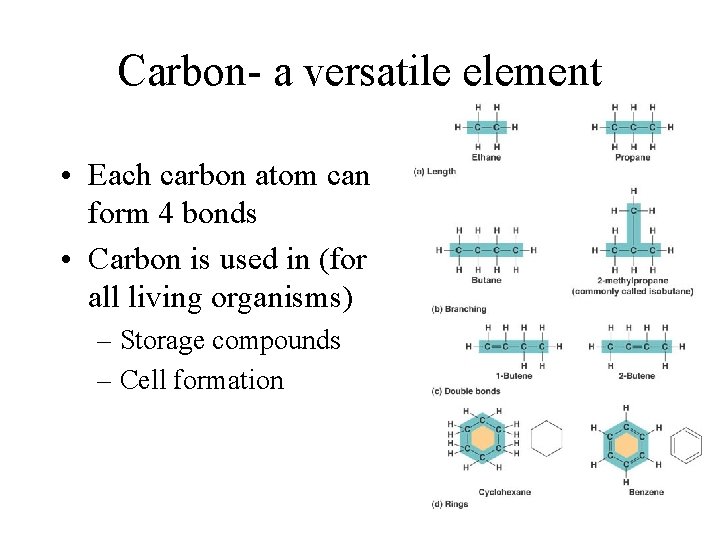
Carbon- a versatile element • Each carbon atom can form 4 bonds • Carbon is used in (for all living organisms) – Storage compounds – Cell formation
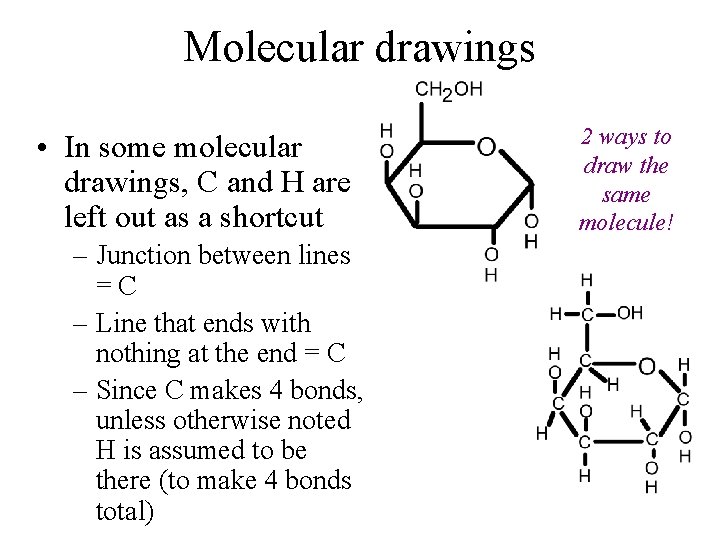
Molecular drawings • In some molecular drawings, C and H are left out as a shortcut – Junction between lines =C – Line that ends with nothing at the end = C – Since C makes 4 bonds, unless otherwise noted H is assumed to be there (to make 4 bonds total) 2 ways to draw the same molecule!
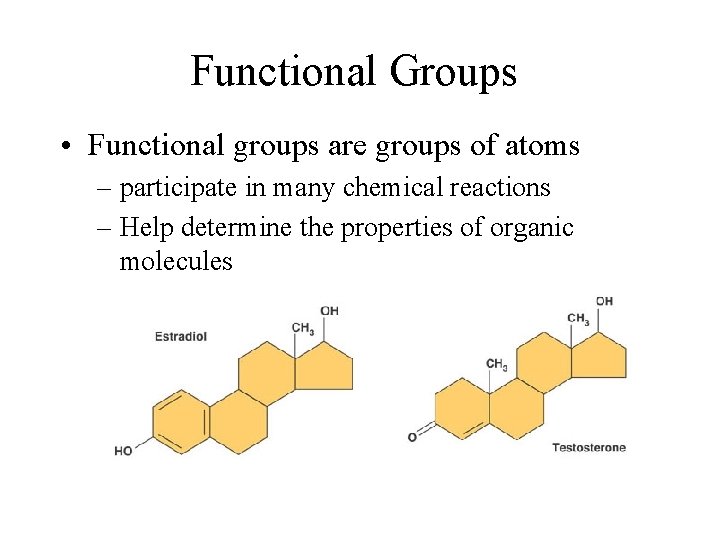
Functional Groups • Functional groups are groups of atoms – participate in many chemical reactions – Help determine the properties of organic molecules

Functional groups • Alcohol (hydroxyl) – Other forms: HO— • Aldehyde – Other forms: -COH – Properties: Polar, Soluble in water • Ketone O – Other forms: CH 2 C CH 2 – Properties: Polar, Soluble in water

Functional groups • Carboxyl – Other forms: -COOH, -COO– Properties: Acidic, Polar, Water Soluble • Amino – Other forms: -NH 2, - NH 3+ – Properties: Basic, Polar
Functional groups • Thiol – Other forms: -SH – Properties: Polar, forms disulfide bridges/bonds in proteins • Phosphate – Other forms: -PO 42– Properties: Charged, Polar
- Slides: 19