Chemistry and Thermodynamics Physics Helps us understands chemistry








- Slides: 33

Chemistry and Thermodynamics • Physics Helps us understands chemistry (and biology etc. )

First Law of Thermodynamics • Energy cannot be created nor destroyed. • Therefore, the total energy of the universe is a constant. • Energy can, however, be converted from one form to another or transferred from a system to the surroundings or vice versa. • But there is more going on than just the First Law © 2009, Prentice-Hall, Inc.

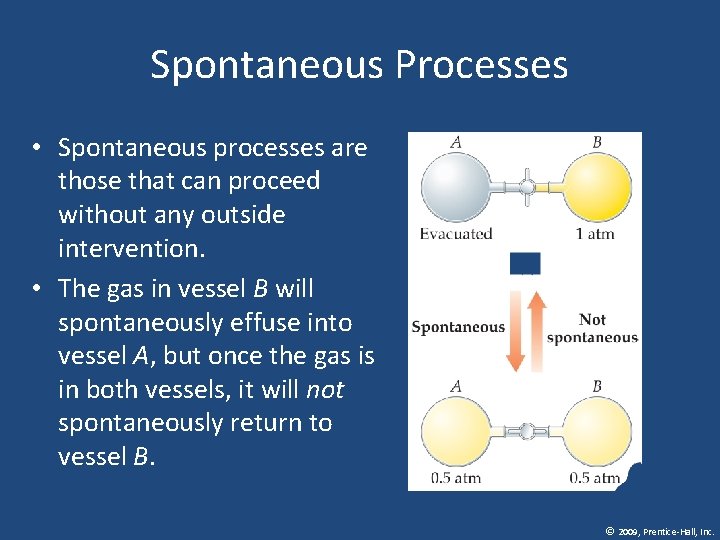
Spontaneous Processes • Spontaneous processes are those that can proceed without any outside intervention. • The gas in vessel B will spontaneously effuse into vessel A, but once the gas is in both vessels, it will not spontaneously return to vessel B. © 2009, Prentice-Hall, Inc.

Spontaneous Processes that are spontaneous in one direction are nonspontaneous in the reverse direction. © 2009, Prentice-Hall, Inc.

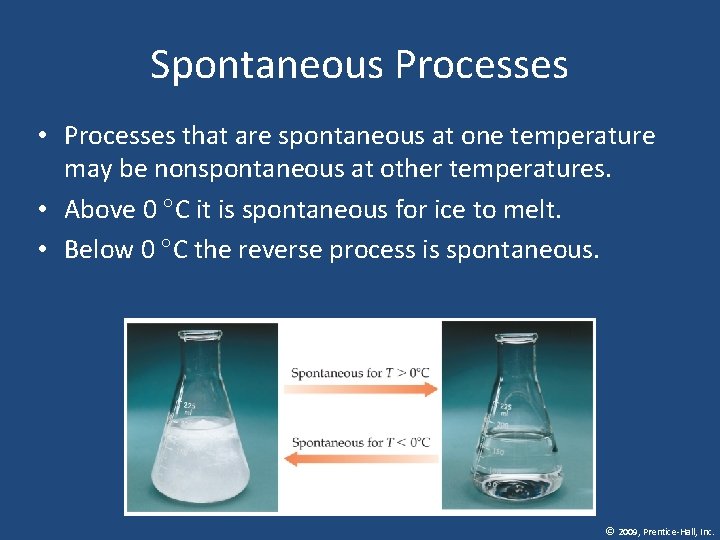
Spontaneous Processes • Processes that are spontaneous at one temperature may be nonspontaneous at other temperatures. • Above 0 C it is spontaneous for ice to melt. • Below 0 C the reverse process is spontaneous. © 2009, Prentice-Hall, Inc.

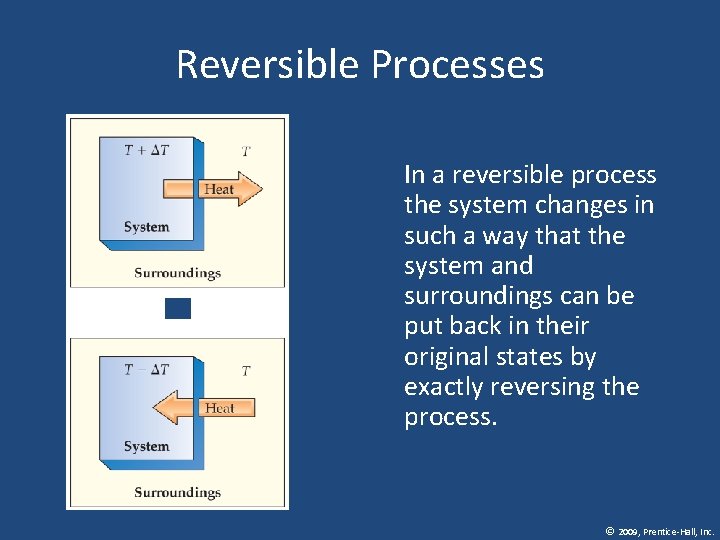
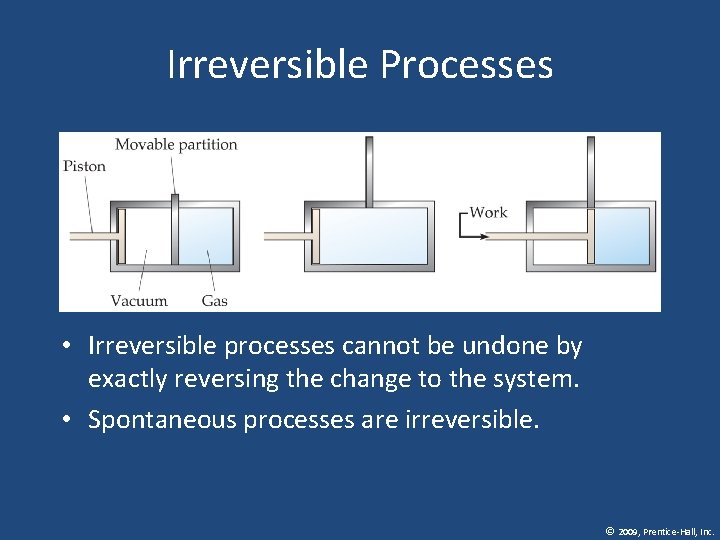
Reversible Processes In a reversible process the system changes in such a way that the system and surroundings can be put back in their original states by exactly reversing the process. © 2009, Prentice-Hall, Inc.

Irreversible Processes • Irreversible processes cannot be undone by exactly reversing the change to the system. • Spontaneous processes are irreversible. © 2009, Prentice-Hall, Inc.

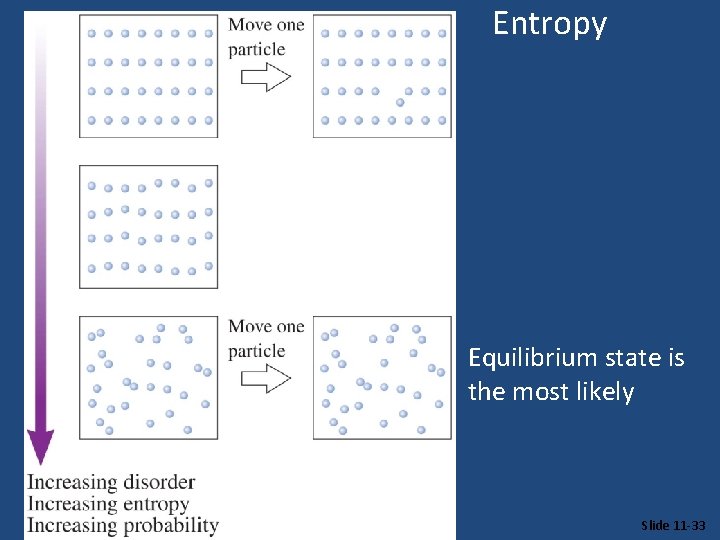
Entropy Equilibrium state is the most likely Slide 11 -33

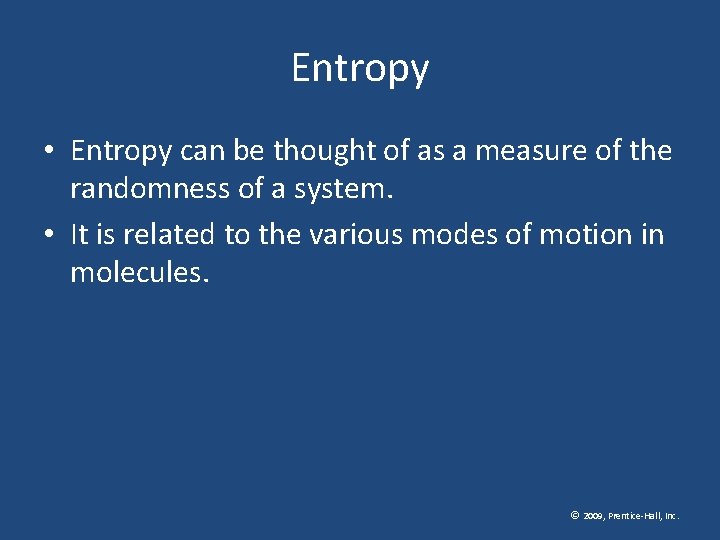
Entropy • Entropy can be thought of as a measure of the randomness of a system. • It is related to the various modes of motion in molecules. © 2009, Prentice-Hall, Inc.

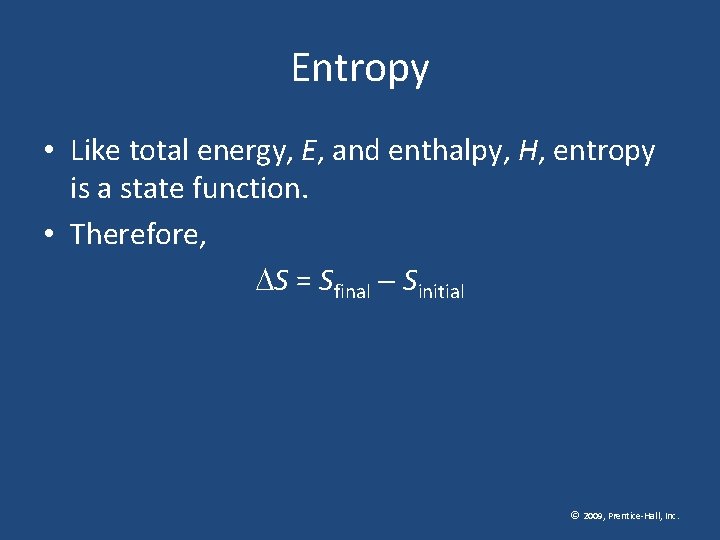
Entropy • Like total energy, E, and enthalpy, H, entropy is a state function. • Therefore, S = Sfinal Sinitial © 2009, Prentice-Hall, Inc.

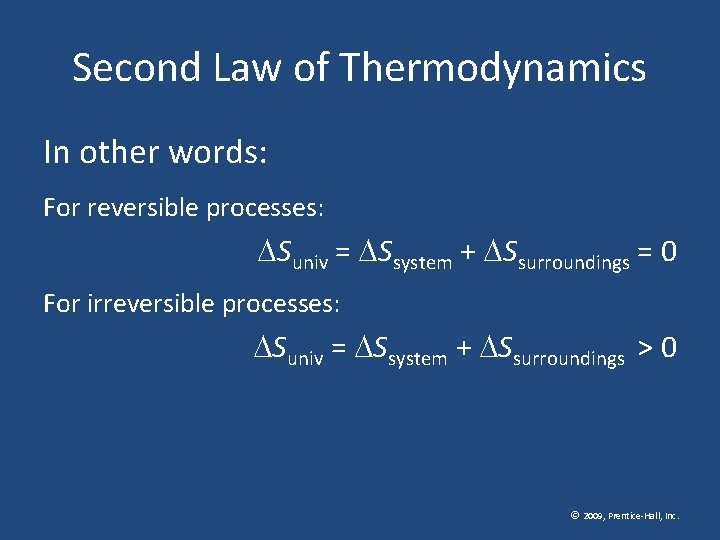
Second Law of Thermodynamics The second law of thermodynamics states that the entropy of the universe increases for spontaneous processes, and the entropy of the universe does not change for reversible processes. © 2009, Prentice-Hall, Inc.

Second Law of Thermodynamics In other words: For reversible processes: Suniv = Ssystem + Ssurroundings = 0 For irreversible processes: Suniv = Ssystem + Ssurroundings > 0 © 2009, Prentice-Hall, Inc.

Second Law of Thermodynamics These last truths mean that as a result of all spontaneous processes the entropy of the universe increases. © 2009, Prentice-Hall, Inc.

Entropy on the Molecular Scale • The number of microstates and, therefore, the entropy tends to increase with increases in – Temperature. – Volume. – The number of independently moving molecules. © 2009, Prentice-Hall, Inc.

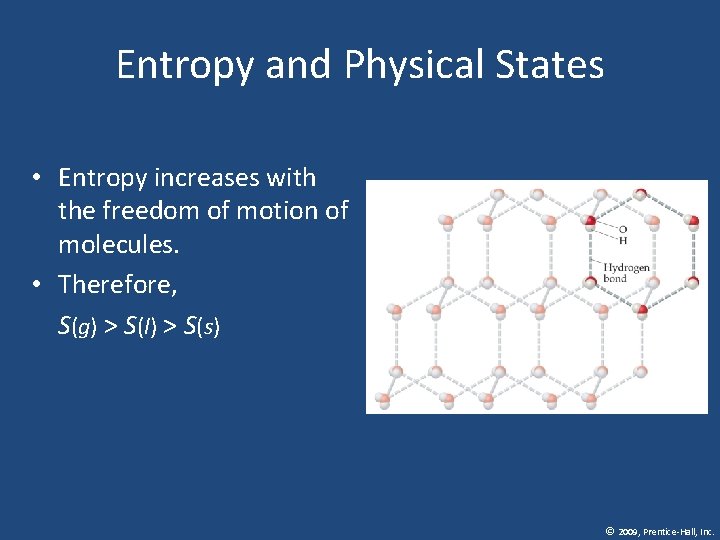
Entropy and Physical States • Entropy increases with the freedom of motion of molecules. • Therefore, S(g) > S(l) > S(s) © 2009, Prentice-Hall, Inc.

Solutions Generally, when a solid is dissolved in a solvent, entropy increases. © 2009, Prentice-Hall, Inc.

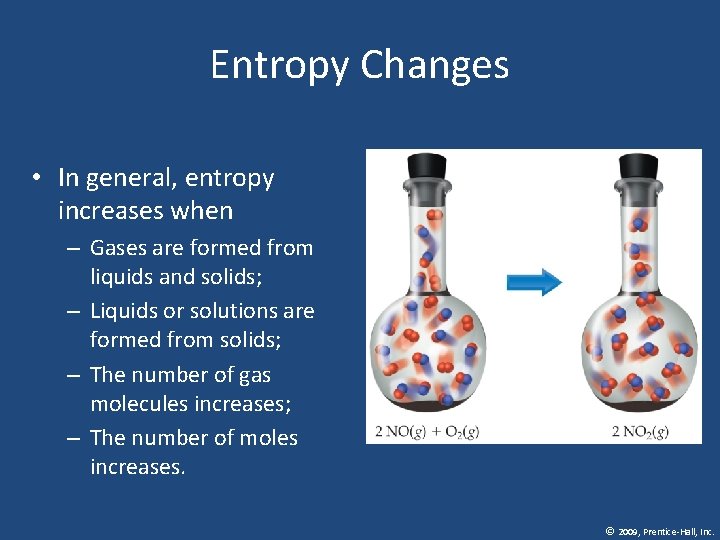
Entropy Changes • In general, entropy increases when – Gases are formed from liquids and solids; – Liquids or solutions are formed from solids; – The number of gas molecules increases; – The number of moles increases. © 2009, Prentice-Hall, Inc.

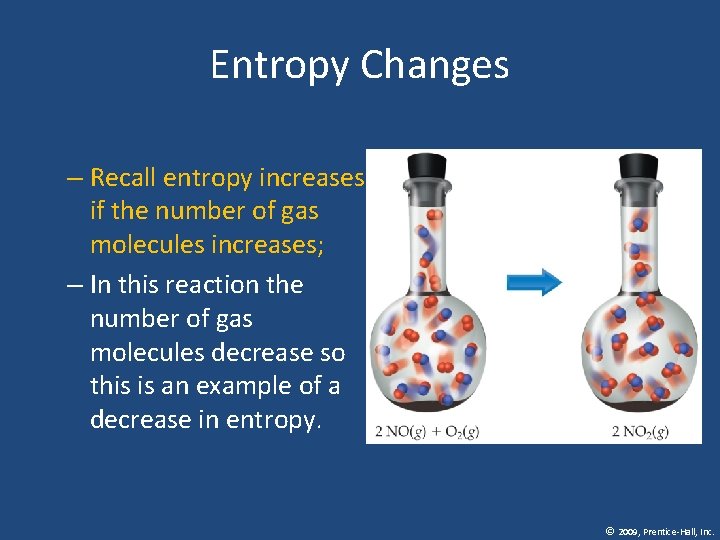
Entropy Changes – Recall entropy increases if the number of gas molecules increases; – In this reaction the number of gas molecules decrease so this is an example of a decrease in entropy. © 2009, Prentice-Hall, Inc.

Entropy decreases when: a. b. c. d. gases are formed from liquids are formed from solids are formed from gases. the number of gas molecules increases during a chemical reaction.

Entropy decreases when: a. b. c. d. gases are formed from liquids are formed from solids are formed from gases. the number of gas molecules increases during a chemical reaction.

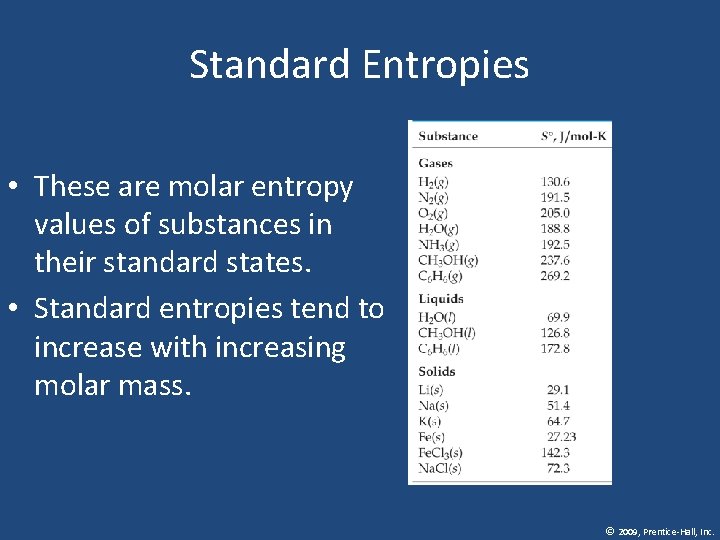
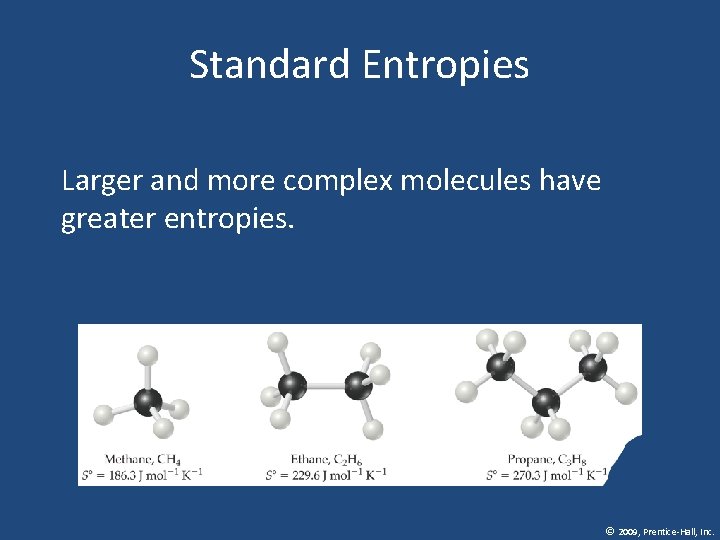
Standard Entropies • These are molar entropy values of substances in their standard states. • Standard entropies tend to increase with increasing molar mass. © 2009, Prentice-Hall, Inc.

Standard Entropies Larger and more complex molecules have greater entropies. © 2009, Prentice-Hall, Inc.

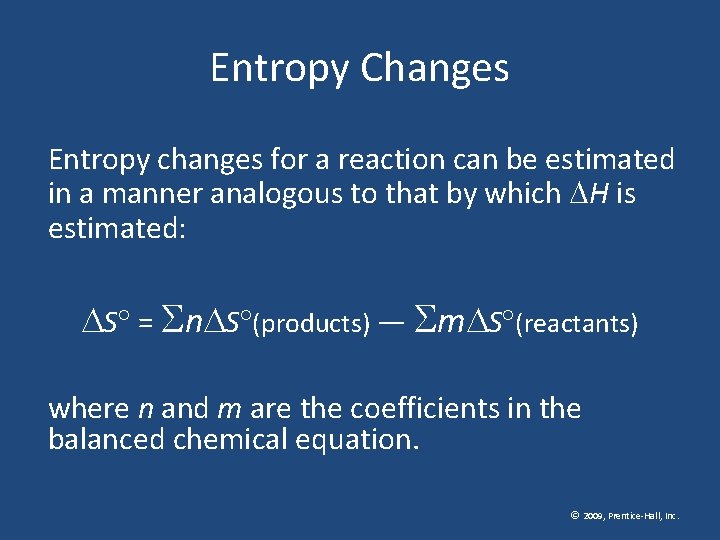
Entropy Changes Entropy changes for a reaction can be estimated in a manner analogous to that by which H is estimated: S = n S (products) — m S (reactants) where n and m are the coefficients in the balanced chemical equation. © 2009, Prentice-Hall, Inc.

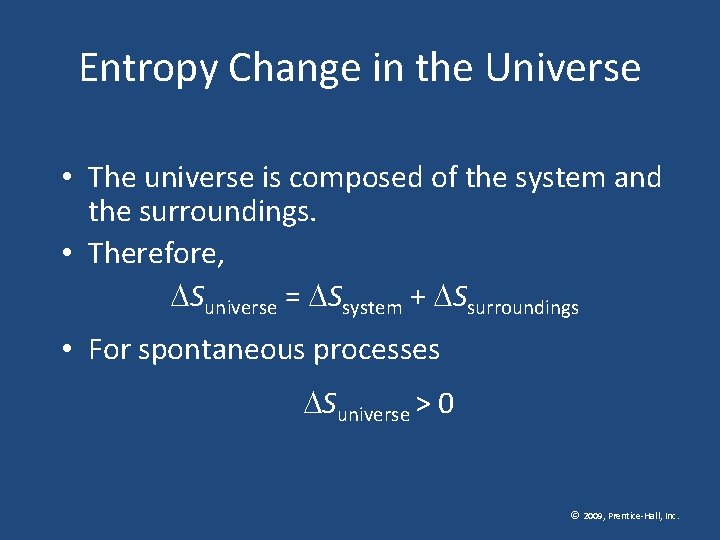
Entropy Change in the Universe • The universe is composed of the system and the surroundings. • Therefore, Suniverse = Ssystem + Ssurroundings • For spontaneous processes Suniverse > 0 © 2009, Prentice-Hall, Inc.

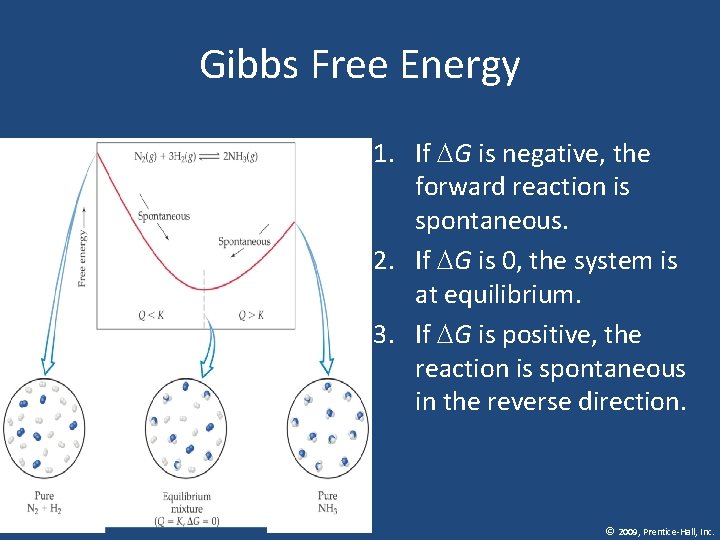
Gibbs Free Energy • Gibbs Free Energy is a quantity that helps tell us whether a process is spontaneous • G= H-TS • G = H T S • When ΔG is negative Suniverse is positive, • Therefore, when G is negative, a process is spontaneous. © 2009, Prentice-Hall, Inc.

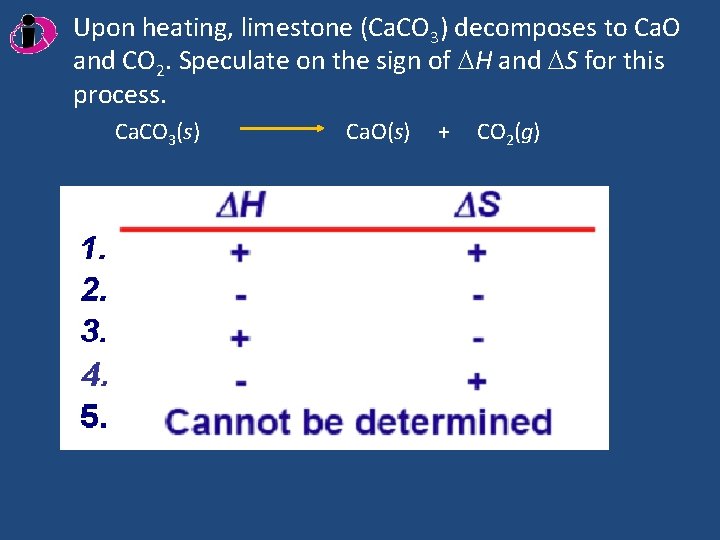
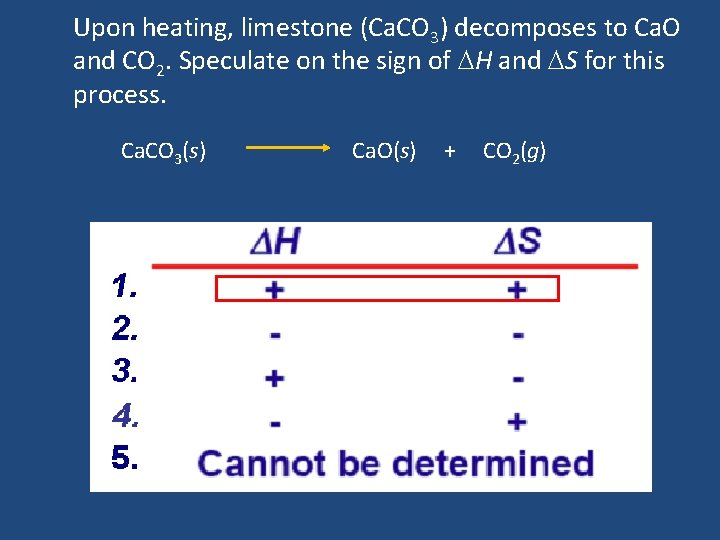
Upon heating, limestone (Ca. CO 3) decomposes to Ca. O and CO 2. Speculate on the sign of H and S for this process. Ca. CO 3(s) Ca. O(s) + CO 2(g)

Upon heating, limestone (Ca. CO 3) decomposes to Ca. O and CO 2. Speculate on the sign of H and S for this process. Ca. CO 3(s) Ca. O(s) + CO 2(g)

Gibbs Free Energy 1. If G is negative, the forward reaction is spontaneous. 2. If G is 0, the system is at equilibrium. 3. If G is positive, the reaction is spontaneous in the reverse direction. © 2009, Prentice-Hall, Inc.

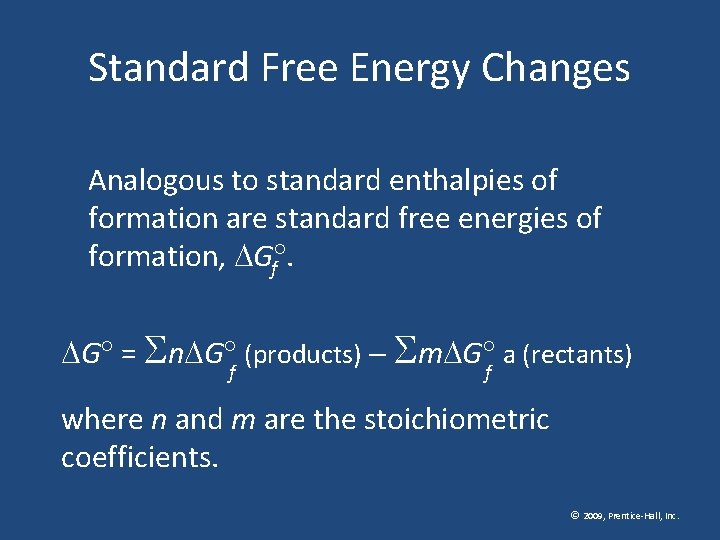
Standard Free Energy Changes Analogous to standard enthalpies of formation are standard free energies of formation, G. f G = n G (products) m G a (rectants) f f where n and m are the stoichiometric coefficients. © 2009, Prentice-Hall, Inc.

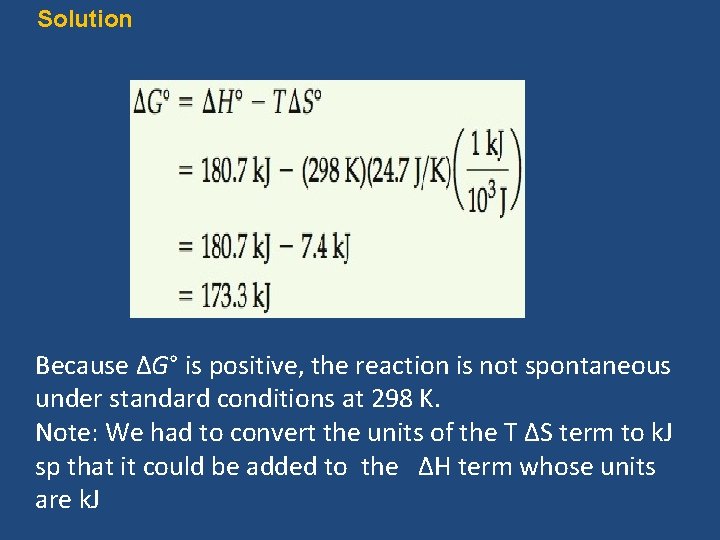
Sample Exercise 19. 6 Calculating Free-Energy Change from ΔH°, T, ΔS° Calculate the standard free energy change for the formation of NO(g) from N 2(g) and O 2(g) at 298 K: N 2(g) + O 2(g) → 2 NO(g) given that ΔH° = 180. 7 k. J and ΔS° = 24. 7 J/K. Is the reaction spontaneous under these circumstances?

Calculate the standard free energy change for the formation of NO(g) from N 2(g) and O 2(g) at 298 K: N 2(g) + O 2(g) → 2 NO(g) given that ΔH° = 180. 7 k. J and ΔS° = 24. 7 J/K. Is the reaction spontaneous under these circumstances? Analyze: We are asked to calculate ΔG° for the indicated reaction (given ΔH°, ΔS° and T) and to predict whether the reaction is spontaneous under standard conditions at 298 K. Plan: To calculate ΔG°, we use Equation, ΔG° = ΔH° – TΔS°. To determine whether the reaction is spontaneous under standard conditions, we look at the sign of ΔG°.

Solution Because ΔG° is positive, the reaction is not spontaneous under standard conditions at 298 K. Note: We had to convert the units of the T ΔS term to k. J sp that it could be added to the ΔH term whose units are k. J

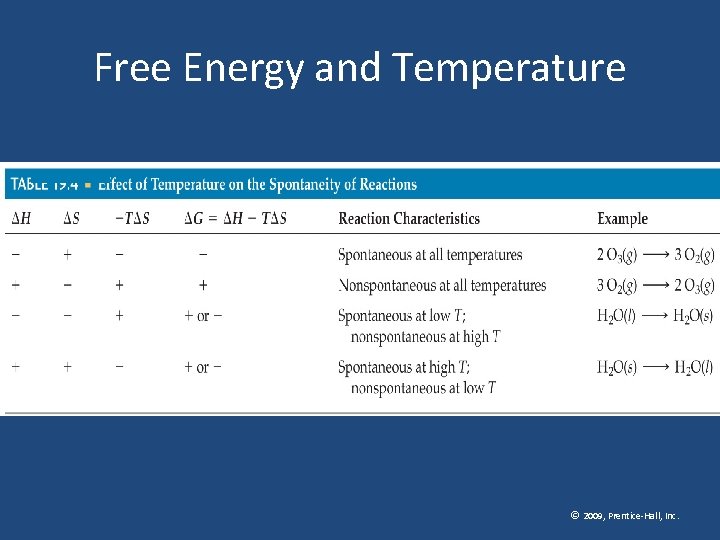
Free Energy and Temperature © 2009, Prentice-Hall, Inc.