Chemistry 30 Unit A Thermochemical Changes Molar Enthalpy


- Slides: 11

Chemistry 30 Unit A: Thermochemical Changes Molar Enthalpy

Ringer! � Why would California Citrus Growers spray their trees with water when cold temperatures threaten?

Potential Energy � In general ◦ Compounds and elements in the gas phase have more potential energy than those same substances in the other phase ◦ This is because molecules in the solid phase are more limited as to position than in gas phase. Their molecules are closer together.

Energy in Chemical Reactions � In chemical reactions, two things occur ◦ Bonds are broken (reactants)– energy required ◦ Bonds are reformed (products) – energy released � If the energy to reform the bonds is greater than the energy to break the bonds of the reactants, you have a n exothermic reaction ◦ The opposite is true for and endothermic reaction

Endothermic & Exothermic Reactions � Endothermic Reaction ◦ 33. 2 KJ + ½N 2 (g) + O 2 (g) → NO 2 (g) � Exothermic Reaction ◦ CH 4 (g) + 2 O 2 (g) → CO 2 (g) + 2 H 2 O (g) + 802. 3 KJ � For energy to be released, there must have been more potential energy in the reactants bonds than the products bonds

Heat content/ Enthalpy (H) � The potential energy stored in the substance � Molar enthalpy (H) ◦ The potential energy in one mol of a substance (KJ/mol)

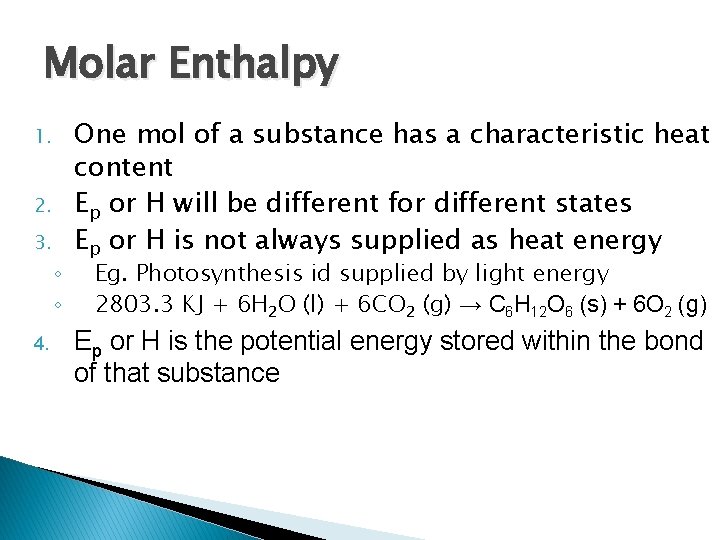
Molar Enthalpy 1. 2. 3. ◦ ◦ 4. One mol of a substance has a characteristic heat content Ep or H will be different for different states Ep or H is not always supplied as heat energy Eg. Photosynthesis id supplied by light energy 2803. 3 KJ + 6 H 2 O (l) + 6 CO 2 (g) → C 6 H 12 O 6 (s) + 6 O 2 (g) Ep or H is the potential energy stored within the bond of that substance

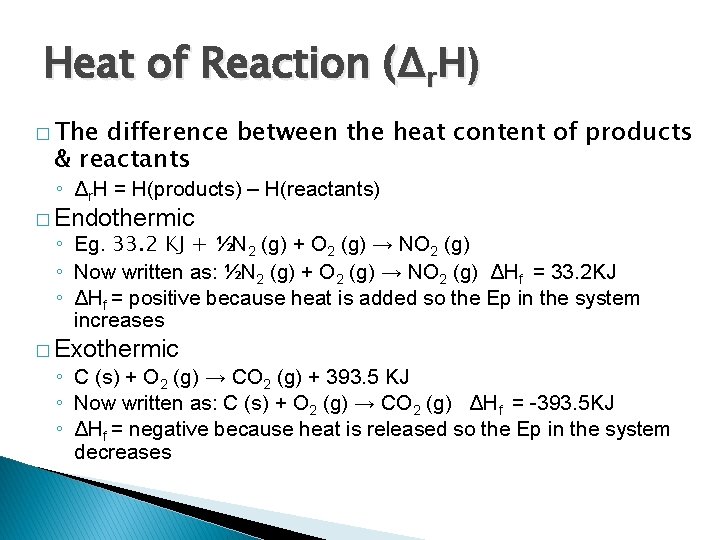
Heat of Reaction (Δr. H) � The difference between the heat content of products & reactants ◦ Δr. H = H(products) – H(reactants) � Endothermic ◦ Eg. 33. 2 KJ + ½N 2 (g) + O 2 (g) → NO 2 (g) ◦ Now written as: ½N 2 (g) + O 2 (g) → NO 2 (g) ΔHf = 33. 2 KJ ◦ ΔHf = positive because heat is added so the Ep in the system increases � Exothermic ◦ C (s) + O 2 (g) → CO 2 (g) + 393. 5 KJ ◦ Now written as: C (s) + O 2 (g) → CO 2 (g) ΔHf = -393. 5 KJ ◦ ΔHf = negative because heat is released so the Ep in the system decreases

Potential Energy Diagrams � Copy SNAP pages 94 -95 for students � Theoretical description of energy changes based on the concept that enthalpy change is an increase or decrease in Ep ◦ ◦ Include states of matter Endothermic = gain energy Exothermic = lose energy Show enthalpy change with correct sign

Example. SNAP pg 96 #2 � For the combustion of methanol, the heat of combustion is -638. 1 KJ per mol of methanol burned. ◦ Write a balanced equation for the reaction using ΔH notation ◦ Sketch a potential energy diagram

Assignment � Reading ◦ Nelson Pages 487 – 499 � Questions ◦ SNAP pages 97 – 98 #’s 1, 3, 4, 5, 7 ◦ Nelson Pg 501 # 2 ◦ Nelson Pg 494 # 3