Chemistry 30 Electrochemical Changes Oxidation Numbers Up Until
Chemistry 30 Electrochemical Changes Oxidation Numbers

Up Until Now … • Redox Reactions ▫ Have been fairly straight forward for single atoms and monatomic ions ▫ One loses the electron and one gains it • A different process is required to keep track of electron transfers in more complex redox reactions
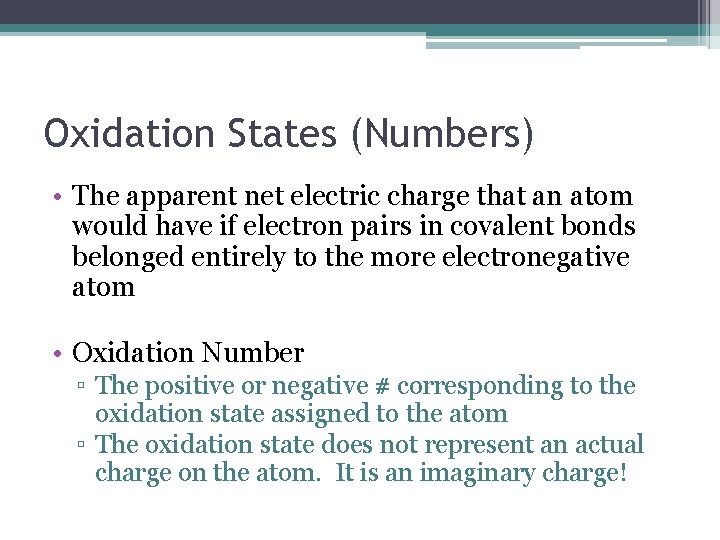
Oxidation States (Numbers) • The apparent net electric charge that an atom would have if electron pairs in covalent bonds belonged entirely to the more electronegative atom • Oxidation Number ▫ The positive or negative # corresponding to the oxidation state assigned to the atom ▫ The oxidation state does not represent an actual charge on the atom. It is an imaginary charge!
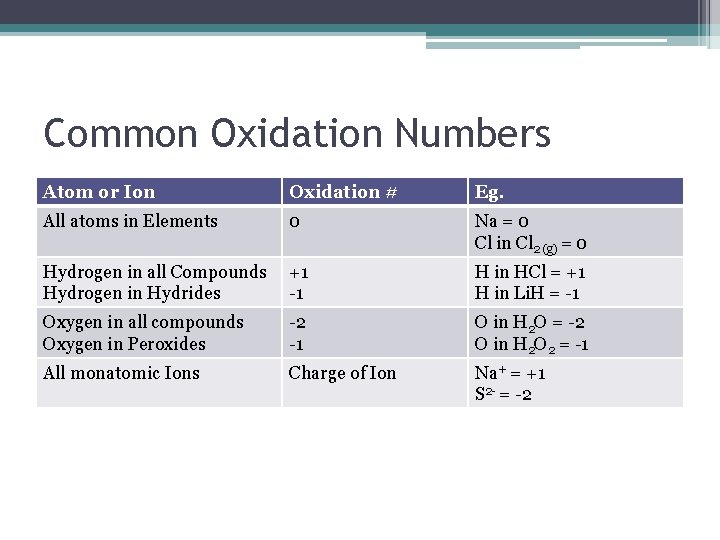
Common Oxidation Numbers Atom or Ion Oxidation # Eg. All atoms in Elements 0 Na = 0 Cl in Cl 2 (g) = 0 Hydrogen in all Compounds Hydrogen in Hydrides +1 -1 H in HCl = +1 H in Li. H = -1 Oxygen in all compounds Oxygen in Peroxides -2 -1 O in H 2 O = -2 O in H 2 O 2 = -1 All monatomic Ions Charge of Ion Na+ = +1 S 2 - = -2

Oxidation Number Rules 1) Oxidation Numbers are per atom and are written below each atom in the formula unit 2) Total oxidation numbers must add up to the charge on the substance ▫ Eg. Cl. O 4 - must have charge of -1

Oxidation Number Rules 3) The basis for oxidation numbers is electronegativities. The atom in the substance with the larger electronegativity win the electron battle thus using it normal charge. ▫ Eg. OF 2 – Flourine has strongest electroneg so O = +2
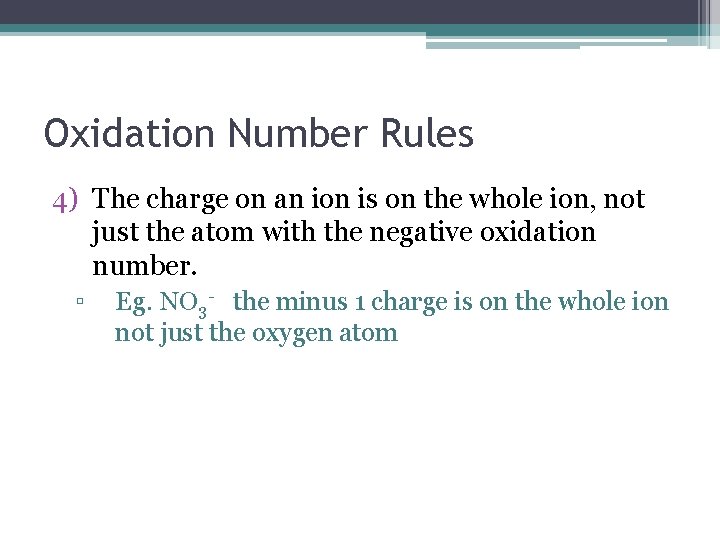
Oxidation Number Rules 4) The charge on an ion is on the whole ion, not just the atom with the negative oxidation number. ▫ Eg. NO 3 - the minus 1 charge is on the whole ion not just the oxygen atom
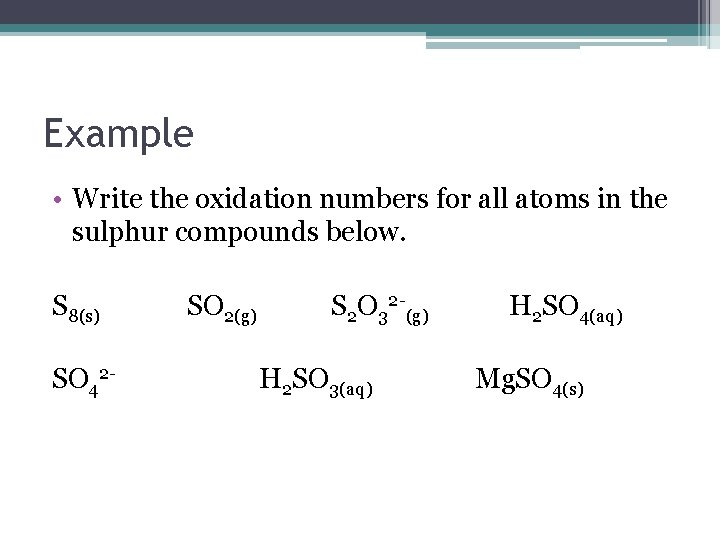
Example • Write the oxidation numbers for all atoms in the sulphur compounds below. S 8(s) SO 42 - SO 2(g) S 2 O 32 -(g) H 2 SO 3(aq) H 2 SO 4(aq) Mg. SO 4(s)

Assignment • Reading ▫ Nelson Pages 583 -588 • Questions ▫ Nelson Page 585 #’s 1 -5 ▫ SNAP Page 337 #’s 1 -2
- Slides: 9