Chemistry 30 Electrochemical Changes Building a Redox Table
Chemistry 30 Electrochemical Changes Building a Redox Table

Redox Possibilities • We talked about the reaction between Cu (s) & Ag+(aq) • Two possibilities exist ▫ Cu(s) + could lose 2 e- to form Cu 2+(aq) while each Ag+ gains an e- to form Ag (s) or … ▫ If Cu 2+ already existed, it could gain 2 e- to form Cu (s) while Ag(s) lost an e- each to form 2 Ag+ ▫ Depends on the two substances involved ▫ Which substance has the stronger tendency to take/lose electrons?

Redox Table – SNAP Pg 305 • Hand out copies to students ▫ Places many common half reactions in order of strongest oxidizing agents and reducing agents ▫ Go through table ▫ Which are more likely to gain or lose electrons? ▫ Which are more likely to cause electrons to be gained or lost?

Spontaneity of Redox reactions • Spontaneous reactions occur without a continuous supply of energy to the system. • Redox spontaneity rule: ▫ A spontaneous redox reaction only occurs if the oxidizing agent is above the reducing agent in the table of redox half reactions

Building a Redox Table • Based on gained electrons ▫ To be done if not given a table • Basically, you compare reactions betweeen two substances and see which one gains electrons. This is your Stronger reducing agent (SRA) ▫ Continue ranking reactions until you figure out the table
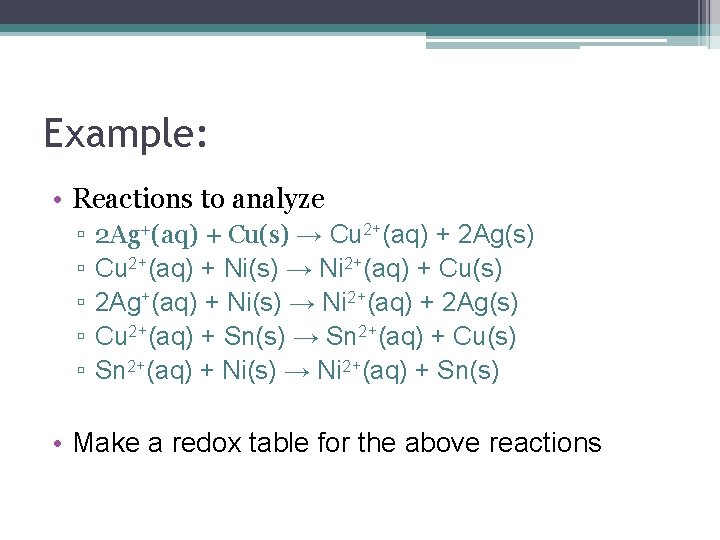
Example: • Reactions to analyze ▫ ▫ ▫ 2 Ag+(aq) + Cu(s) → Cu 2+(aq) + 2 Ag(s) Cu 2+(aq) + Ni(s) → Ni 2+(aq) + Cu(s) 2 Ag+(aq) + Ni(s) → Ni 2+(aq) + 2 Ag(s) Cu 2+(aq) + Sn(s) → Sn 2+(aq) + Cu(s) Sn 2+(aq) + Ni(s) → Ni 2+(aq) + Sn(s) • Make a redox table for the above reactions

Trial & Error • The redox table ▫ Was built by trial and error over years of reactions ▫ Imagine how long it would take to create the SNAP table you have

Example • Go through # 1 and #2 on SNAP page 306 • Make copies for students

Assignment • Reading ▫ Nelson Pages 569 – 574 • Questions ▫ SNAP Page 307 -308 #’s 1 -4, 7, 8 ▫ Nelson Page 573 -574 #’s 12, 20
- Slides: 9