CHEMISTRY 3 ELEMENTS ACIDS AND WATER ENERGY AND






- Slides: 31

CHEMISTRY 3 ELEMENTS, ACIDS AND WATER & ENERGY AND CHEMICAL TESTS

ELEMENTS, ACIDS & WATER

Periodic Table History Early 1800 s • there were only two ways to categorize elements: physical and chemical properties & relative atomic mass • nothing was known about atomic structure, protons or electron so there was no such thing as atomic number • so the known elements were arranged in order of atomic mass • when this was done a periodic pattern was noticed in the properties of the elements Newland’s Law of Octaves • he gave the first food try at arranging the elements in 1864 • he noticed that every eighth element had similar properties so he listed some of the known elements in rows of seven • these sets of eights were Newlands Octaves but the pattern broke down on the third row as the transition metals messed it up • he left no gaps so his worked was ignored but he was quite close • his work was criticized because: • groups contained elements that didn’t have similar properties • metals and non metals were mixed up • gaps weren’t left for elements that hadn’t been discovered yet

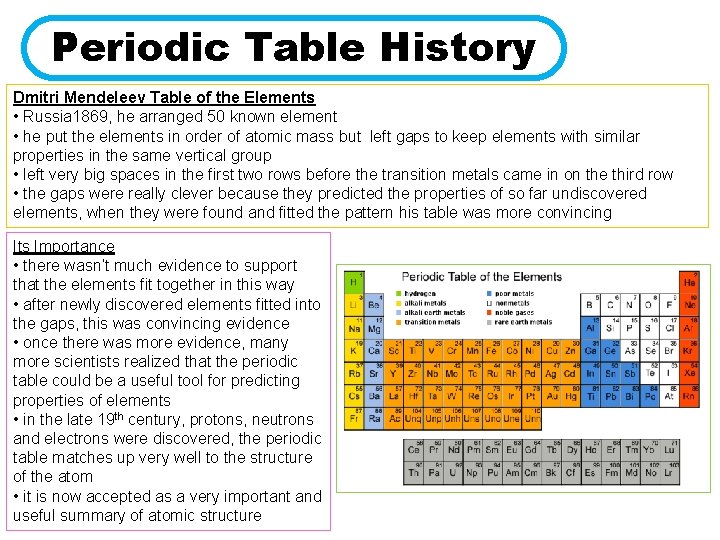
Periodic Table History Dmitri Mendeleev Table of the Elements • Russia 1869, he arranged 50 known element • he put the elements in order of atomic mass but left gaps to keep elements with similar properties in the same vertical group • left very big spaces in the first two rows before the transition metals came in on the third row • the gaps were really clever because they predicted the properties of so far undiscovered elements, when they were found and fitted the pattern his table was more convincing Its Importance • there wasn’t much evidence to support that the elements fit together in this way • after newly discovered elements fitted into the gaps, this was convincing evidence • once there was more evidence, many more scientists realized that the periodic table could be a useful tool for predicting properties of elements • in the late 19 th century, protons, neutrons and electrons were discovered, the periodic table matches up very well to the structure of the atom • it is now accepted as a very important and useful summary of atomic structure

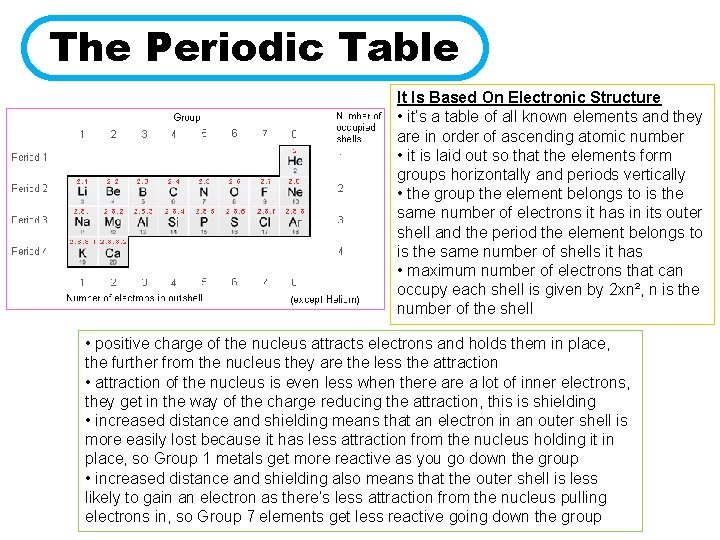
The Periodic Table It Is Based On Electronic Structure • it’s a table of all known elements and they are in order of ascending atomic number • it is laid out so that the elements form groups horizontally and periods vertically • the group the element belongs to is the same number of electrons it has in its outer shell and the period the element belongs to is the same number of shells it has • maximum number of electrons that can occupy each shell is given by 2 xn², n is the number of the shell • positive charge of the nucleus attracts electrons and holds them in place, the further from the nucleus they are the less the attraction • attraction of the nucleus is even less when there a lot of inner electrons, they get in the way of the charge reducing the attraction, this is shielding • increased distance and shielding means that an electron in an outer shell is more easily lost because it has less attraction from the nucleus holding it in place, so Group 1 metals get more reactive as you go down the group • increased distance and shielding also means that the outer shell is less likely to gain an electron as there’s less attraction from the nucleus pulling electrons in, so Group 7 elements get less reactive going down the group

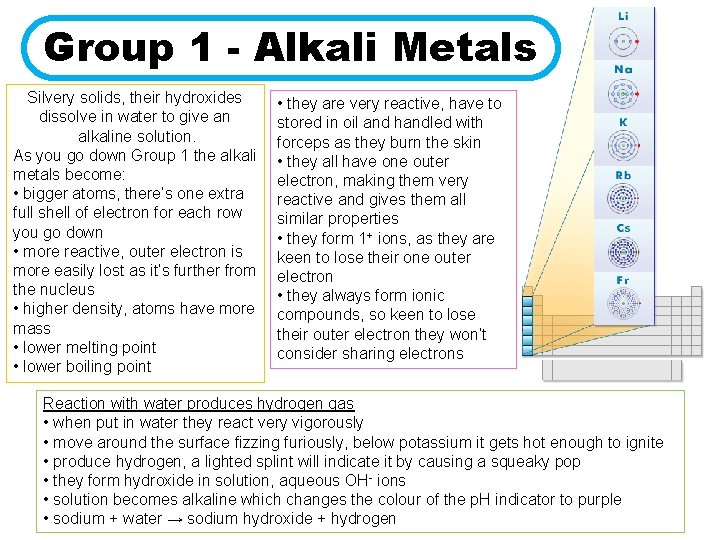
Group 1 - Alkali Metals Silvery solids, their hydroxides dissolve in water to give an alkaline solution. As you go down Group 1 the alkali metals become: • bigger atoms, there’s one extra full shell of electron for each row you go down • more reactive, outer electron is more easily lost as it’s further from the nucleus • higher density, atoms have more mass • lower melting point • lower boiling point • they are very reactive, have to stored in oil and handled with forceps as they burn the skin • they all have one outer electron, making them very reactive and gives them all similar properties • they form 1+ ions, as they are keen to lose their one outer electron • they always form ionic compounds, so keen to lose their outer electron they won’t consider sharing electrons Reaction with water produces hydrogen gas • when put in water they react very vigorously • move around the surface fizzing furiously, below potassium it gets hot enough to ignite • produce hydrogen, a lighted splint will indicate it by causing a squeaky pop • they form hydroxide in solution, aqueous OH- ions • solution becomes alkaline which changes the colour of the p. H indicator to purple • sodium + water → sodium hydroxide + hydrogen

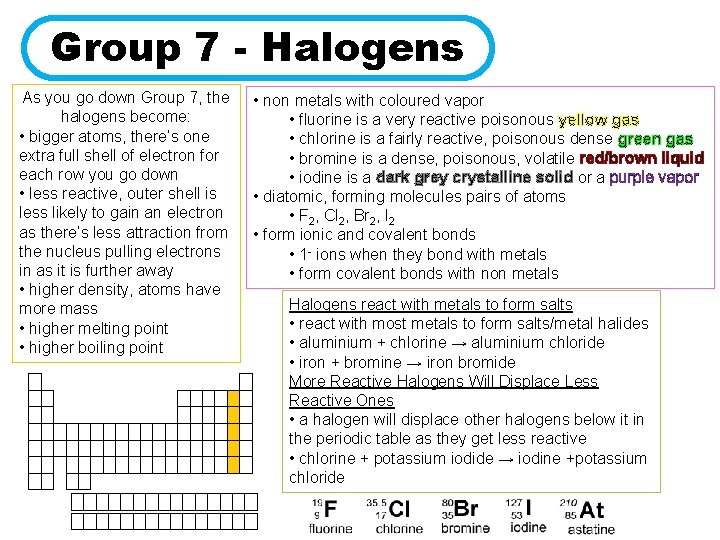
Group 7 - Halogens As you go down Group 7, the halogens become: • bigger atoms, there’s one extra full shell of electron for each row you go down • less reactive, outer shell is less likely to gain an electron as there’s less attraction from the nucleus pulling electrons in as it is further away • higher density, atoms have more mass • higher melting point • higher boiling point • non metals with coloured vapor • fluorine is a very reactive poisonous yellow gas • chlorine is a fairly reactive, poisonous dense green gas • bromine is a dense, poisonous, volatile red/brown liquid • iodine is a dark grey crystalline solid or a purple vapor • diatomic, forming molecules pairs of atoms • F 2, Cl 2, Br 2, I 2 • form ionic and covalent bonds • 1 - ions when they bond with metals • form covalent bonds with non metals Halogens react with metals to form salts • react with most metals to form salts/metal halides • aluminium + chlorine → aluminium chloride • iron + bromine → iron bromide More Reactive Halogens Will Displace Less Reactive Ones • a halogen will displace other halogens below it in the periodic table as they get less reactive • chlorine + potassium iodide → iodine +potassium chloride

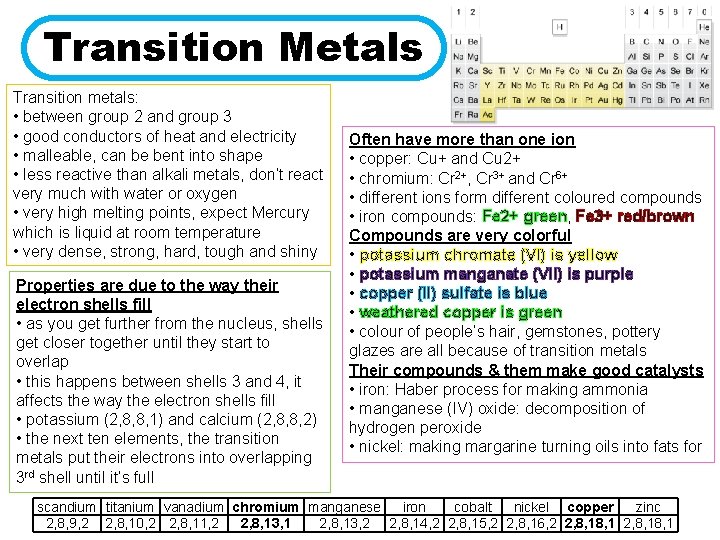
Transition Metals Transition metals: • between group 2 and group 3 • good conductors of heat and electricity • malleable, can be bent into shape • less reactive than alkali metals, don’t react very much with water or oxygen • very high melting points, expect Mercury which is liquid at room temperature • very dense, strong, hard, tough and shiny Properties are due to the way their electron shells fill • as you get further from the nucleus, shells get closer together until they start to overlap • this happens between shells 3 and 4, it affects the way the electron shells fill • potassium (2, 8, 8, 1) and calcium (2, 8, 8, 2) • the next ten elements, the transition metals put their electrons into overlapping 3 rd shell until it’s full Often have more than one ion • copper: Cu+ and Cu 2+ • chromium: Cr 2+, Cr 3+ and Cr 6+ • different ions form different coloured compounds • iron compounds: Fe 2+ green, Fe 3+ red/brown Compounds are very colorful • potassium chromate (VI) is yellow • potassium manganate (VII) is purple • copper (II) sulfate is blue • weathered copper is green • colour of people’s hair, gemstones, pottery glazes are all because of transition metals Their compounds & them make good catalysts • iron: Haber process for making ammonia • manganese (IV) oxide: decomposition of hydrogen peroxide • nickel: making margarine turning oils into fats for scandium titanium vanadium chromium manganese iron cobalt nickel copper zinc 2, 8, 9, 2 2, 8, 10, 2 2, 8, 11, 2 2, 8, 13, 1 2, 8, 13, 2 2, 8, 14, 2 2, 8, 15, 2 2, 8, 16, 2 2, 8, 18, 1

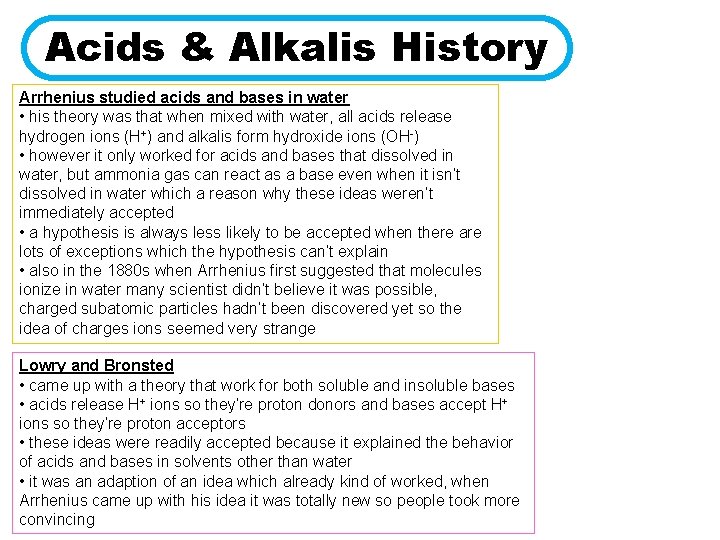
Acids & Alkalis History Arrhenius studied acids and bases in water • his theory was that when mixed with water, all acids release hydrogen ions (H+) and alkalis form hydroxide ions (OH-) • however it only worked for acids and bases that dissolved in water, but ammonia gas can react as a base even when it isn’t dissolved in water which a reason why these ideas weren’t immediately accepted • a hypothesis is always less likely to be accepted when there are lots of exceptions which the hypothesis can’t explain • also in the 1880 s when Arrhenius first suggested that molecules ionize in water many scientist didn’t believe it was possible, charged subatomic particles hadn’t been discovered yet so the idea of charges ions seemed very strange Lowry and Bronsted • came up with a theory that work for both soluble and insoluble bases • acids release H+ ions so they’re proton donors and bases accept H+ ions so they’re proton acceptors • these ideas were readily accepted because it explained the behavior of acids and bases in solvents other than water • it was an adaption of an idea which already kind of worked, when Arrhenius came up with his idea it was totally new so people took more convincing

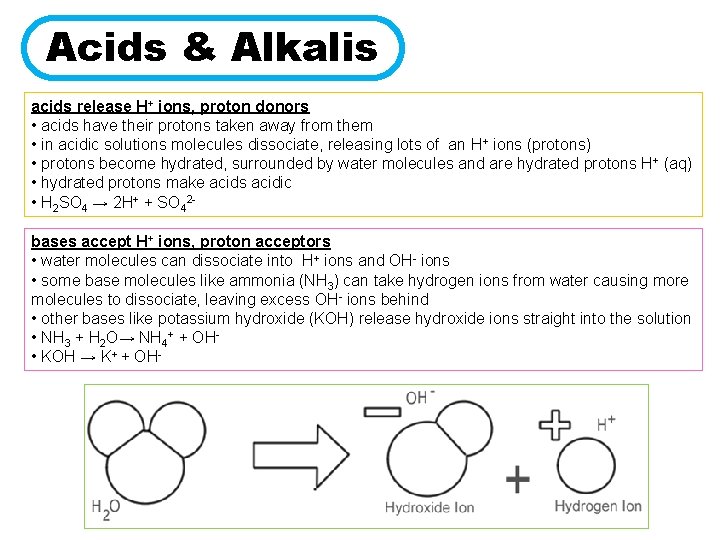
Acids & Alkalis acids release H+ ions, proton donors • acids have their protons taken away from them • in acidic solutions molecules dissociate, releasing lots of an H+ ions (protons) • protons become hydrated, surrounded by water molecules and are hydrated protons H+ (aq) • hydrated protons make acids acidic • H 2 SO 4 → 2 H+ + SO 42 bases accept H+ ions, proton acceptors • water molecules can dissociate into H+ ions and OH- ions • some base molecules like ammonia (NH 3) can take hydrogen ions from water causing more molecules to dissociate, leaving excess OH- ions behind • other bases like potassium hydroxide (KOH) release hydroxide ions straight into the solution • NH 3 + H 2 O→ NH 4+ + OH • KOH → K+ + OH-

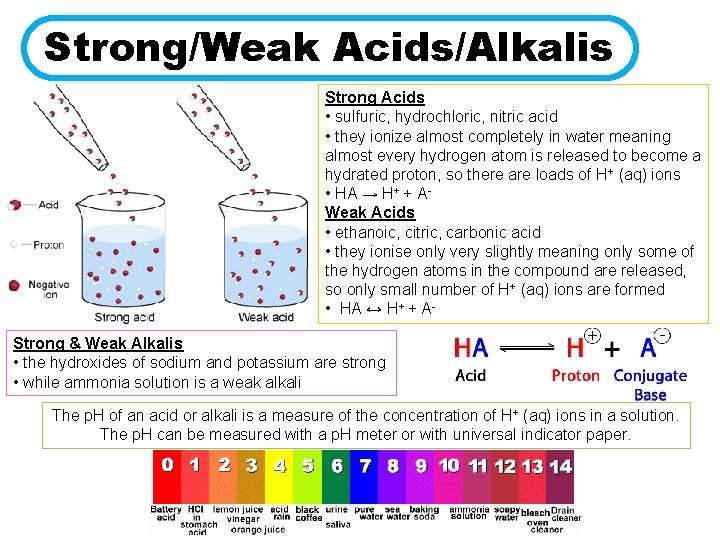
Strong/Weak Acids/Alkalis Strong Acids • sulfuric, hydrochloric, nitric acid • they ionize almost completely in water meaning almost every hydrogen atom is released to become a hydrated proton, so there are loads of H+ (aq) ions • HA → H+ + AWeak Acids • ethanoic, citric, carbonic acid • they ionise only very slightly meaning only some of the hydrogen atoms in the compound are released, so only small number of H+ (aq) ions are formed • HA ↔ H+ + AStrong & Weak Alkalis • the hydroxides of sodium and potassium are strong • while ammonia solution is a weak alkali The p. H of an acid or alkali is a measure of the concentration of H+ (aq) ions in a solution. The p. H can be measured with a p. H meter or with universal indicator paper.

Titrations are used to find out concentrations • they allow you to find out exactly how much acid is needed to neutralise a quantity of alkali and vice versa 1) fill a burette with an alkali/acid solution of known concentration 2) accurately measure an amount of alkali/ acid and place it in the conical flask 3) add an indicator to the flask • strong acid + strong alkali → any indicator • strong acid + weak alkali → methyl orange • weak acid + strong alkali → phenolphthalein 4) slowly add the alkali/acid until the mixture in the flask changes colour • methyl orange: yellow in alkalis, red in acids • phenolphthalein: pink in alkalis, colourless in acids 5) record the amount of alkali/acid used to neutralise the mixture in the flask and repeat process

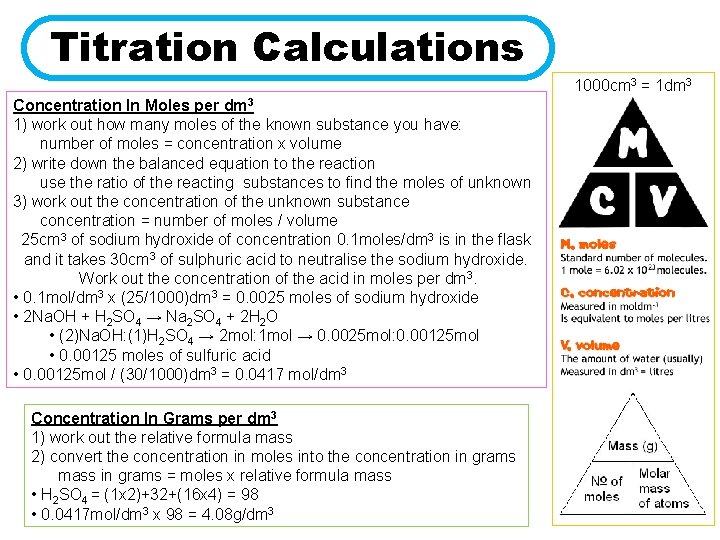
Titration Calculations 1000 cm 3 = 1 dm 3 Concentration In Moles per dm 3 1) work out how many moles of the known substance you have: number of moles = concentration x volume 2) write down the balanced equation to the reaction use the ratio of the reacting substances to find the moles of unknown 3) work out the concentration of the unknown substance concentration = number of moles / volume 25 cm 3 of sodium hydroxide of concentration 0. 1 moles/dm 3 is in the flask and it takes 30 cm 3 of sulphuric acid to neutralise the sodium hydroxide. Work out the concentration of the acid in moles per dm 3. • 0. 1 mol/dm 3 x (25/1000)dm 3 = 0. 0025 moles of sodium hydroxide • 2 Na. OH + H 2 SO 4 → Na 2 SO 4 + 2 H 2 O • (2)Na. OH: (1)H 2 SO 4 → 2 mol: 1 mol → 0. 0025 mol: 0. 00125 mol • 0. 00125 moles of sulfuric acid • 0. 00125 mol / (30/1000)dm 3 = 0. 0417 mol/dm 3 Concentration In Grams per dm 3 1) work out the relative formula mass 2) convert the concentration in moles into the concentration in grams mass in grams = moles x relative formula mass • H 2 SO 4 = (1 x 2)+32+(16 x 4) = 98 • 0. 0417 mol/dm 3 x 98 = 4. 08 g/dm 3

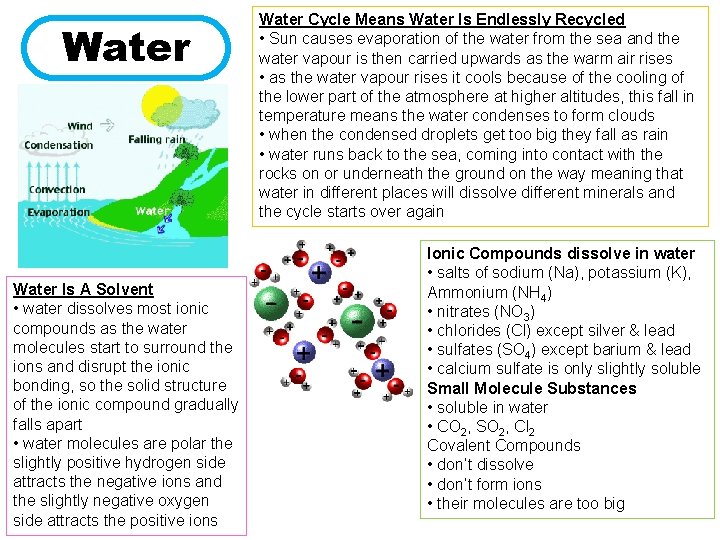
Water Is A Solvent • water dissolves most ionic compounds as the water molecules start to surround the ions and disrupt the ionic bonding, so the solid structure of the ionic compound gradually falls apart • water molecules are polar the slightly positive hydrogen side attracts the negative ions and the slightly negative oxygen side attracts the positive ions Water Cycle Means Water Is Endlessly Recycled • Sun causes evaporation of the water from the sea and the water vapour is then carried upwards as the warm air rises • as the water vapour rises it cools because of the cooling of the lower part of the atmosphere at higher altitudes, this fall in temperature means the water condenses to form clouds • when the condensed droplets get too big they fall as rain • water runs back to the sea, coming into contact with the rocks on or underneath the ground on the way meaning that water in different places will dissolve different minerals and the cycle starts over again Ionic Compounds dissolve in water • salts of sodium (Na), potassium (K), Ammonium (NH 4) • nitrates (NO 3) • chlorides (Cl) except silver & lead • sulfates (SO 4) except barium & lead • calcium sulfate is only slightly soluble Small Molecule Substances • soluble in water • CO 2, SO 2, Cl 2 Covalent Compounds • don’t dissolve • don’t form ions • their molecules are too big

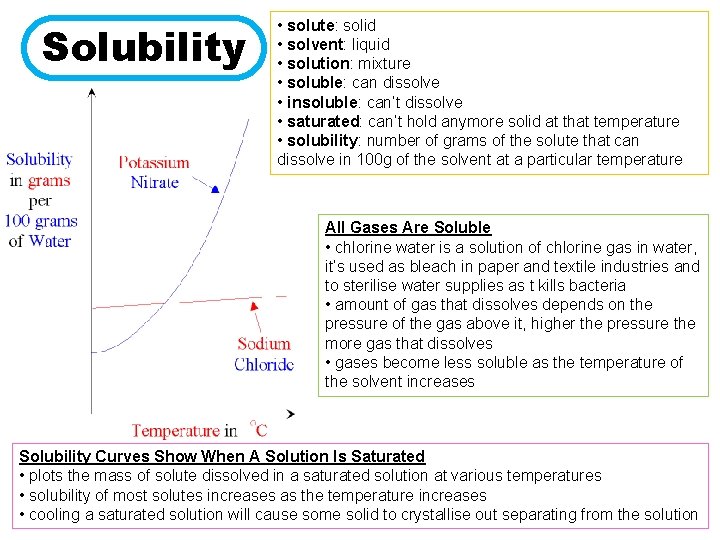
Solubility • solute: solid • solvent: liquid • solution: mixture • soluble: can dissolve • insoluble: can’t dissolve • saturated: can’t hold anymore solid at that temperature • solubility: number of grams of the solute that can dissolve in 100 g of the solvent at a particular temperature All Gases Are Soluble • chlorine water is a solution of chlorine gas in water, it’s used as bleach in paper and textile industries and to sterilise water supplies as t kills bacteria • amount of gas that dissolves depends on the pressure of the gas above it, higher the pressure the more gas that dissolves • gases become less soluble as the temperature of the solvent increases Solubility Curves Show When A Solution Is Saturated • plots the mass of solute dissolved in a saturated solution at various temperatures • solubility of most solutes increases as the temperature increases • cooling a saturated solution will cause some solid to crystallise out separating from the solution

Hard Water Advantages • tastes better • reduces heart illness • provides useful calcium ions • scale forms a protective coating stopping poisonous metal ions getting into drinking water in pipes and protects from rust Disadvantages • more soap is needed to lather • can lead to deposits forming • expensive to remove hardness Hardness Is Caused By Ca 2+ and Mg 2+ Ions • hard water is caused by dissolved calcium and magnesium compounds in water • water passes through rocks like limestone, chalk and gypsum dissolving the minerals • rain falling on some types of rocks can dissolve magnesium sulfate and calcium sulfate • when carbon dioxide from the air dissolves in rain water it forms carbonic acid CO 2 + H 20 → H 2 CO 3 • if there’s calcium carbonate (Ca. CO 3) in the rocks calcium hydrogencarbonate is formed which is soluble and similarly with rock containing Mg. CO 3 H 2 CO 3 + Ca. CO 3 → Ca(HCO 3)2 Causing Scum • scum is formed when soap is added to hard water • sodium stearate (soap) + Ca 2+ (or. Mg 2+) → calcium stearate + Na+ • soap + ions dissolved in water → scum + sodium ion which is dissolved in water • this means to get a decent lather you need to use more soap Causing Scale • scale is formed when water is heated and leaves a deposit, it is also known as lime scale • this happens inside pipes, boilers and kettles causing blockages and them to be less efficient as it is slights a thermal insulator • Ca 2+ (aq) + HCO 3 - (aq) → Ca. CO 3 (s) + (water and carbon dioxide)

Removing Hardness Adding Sodium Carbonate Na. CO 3 • the carbonate ion joins onto the calcium or magnesium ions and make an insoluble precipitate which can be easily removed • hard water + washing soda → scale + soft water • Ca 2+ (aq) + CO 32 - (aq) → Ca. CO 3 Using An Ion Exchange Resin • water passes through a column where calcium and magnesium ions are exchanged for sodium ions, sodium ions don’t cause hardness • resin is a huge insoluble resin molecule • Na 2 Resin (s) + Ca 2+ (aq) → Ca. Resin (s) + 2 Na+ (aq) Removing Scale • scale is mainly calcium carbonate and can be dissolved by an acid • most rescaling products contain some kind of acid

Water Quality • to monitor water quality water companies take samples at each stage • some people are still not satisfied and buy filters that contain carbon or silver to remove substances from their tap water, carbon removes chlorine taste and silver kills bugs • some people in hard water areas buy water softeners which contain ion exchange resins • totally pure water with nothing dissolved can be produced by distillation, boiling water to make steam and condensing the steam Drinking Water Needs To Be Good Quality • water is essential for life • but it must be free of poisonous salts and harmful micro organisms which can cause diseases • most of our drinking water comes from reservoirs • water companies choose to build reservoirs where there’s a good supply of clean water, water flows into them from rivers and groundwater government agencies monitor pollution in reservoirs, rivers and groundwater Water Undergoes Treatment 1) water passes through a mesh screen to remove insoluble solids 2) treated with ozone or chlorine to kill micro organisms 3) chemicals are added to make solids and micro organisms stick together and fall to the bottom 4) water is filtered through gravel beds to remove all the solids, nasty tastes and odours can be removed by passing it through activated carbon filters or with carbon slurry 5) the p. H is corrected if the water is too acidic or too alkaline 6) water is chlorinated to kill off any harmful micro organisms left

ENERGY & CHEMICAL TESTS

Energy Endothermic Reactions takes in energy from the surroundings, the energy required to break old bonds is greater than the energy released when new bonds are formed. Exothermic Reactions gives out energy to the surroundings, the energy released when new bonds are formed is greater than the energy used in breaking old bonds. Energy Transfer Can Be Measured • amount of energy produced by a chemical reaction in a solution can be measured by taking the temperature of the reactants, mixing them and measuring the temperature of the solutions at the end of the reaction • biggest problem with energy measurements is the amount of energy lost to the surroundings • you can reduce this by putting the solution in a polystyrene cup in a beaker of cotton wool to give more insulation and putting a lid on the cup to reduce energy lost by evaporation • this method works for reactions of solids with water and neutralization reactions Breaking and Forming Bonds • during a chemical reaction, old bonds are broken and new bonds are formed • energy must always be supplied to break existing bonds, so bond breaking is an endothermic process • energy is always released when new bonds are formed, so bond formation is an exothermic process • the overall reaction is endothermic or exothermic, depends on which energy supplied or released is greater

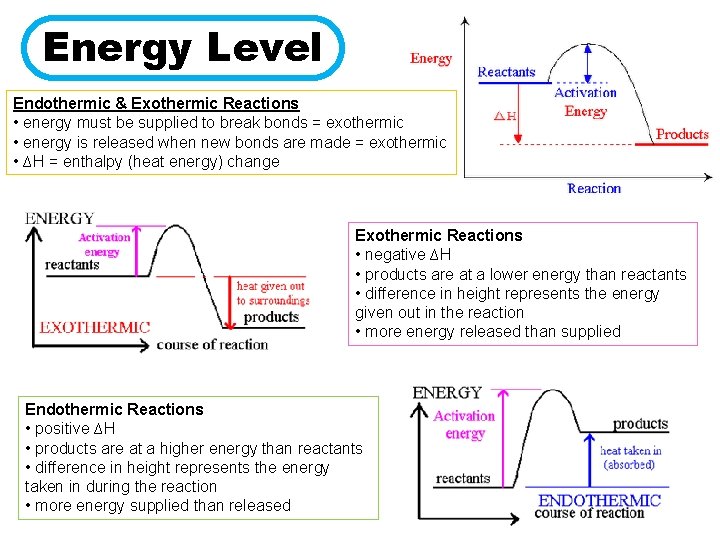
Energy Level Endothermic & Exothermic Reactions • energy must be supplied to break bonds = exothermic • energy is released when new bonds are made = exothermic • ∆H = enthalpy (heat energy) change Exothermic Reactions • negative ∆H • products are at a lower energy than reactants • difference in height represents the energy given out in the reaction • more energy released than supplied Endothermic Reactions • positive ∆H • products are at a higher energy than reactants • difference in height represents the energy taken in during the reaction • more energy supplied than released

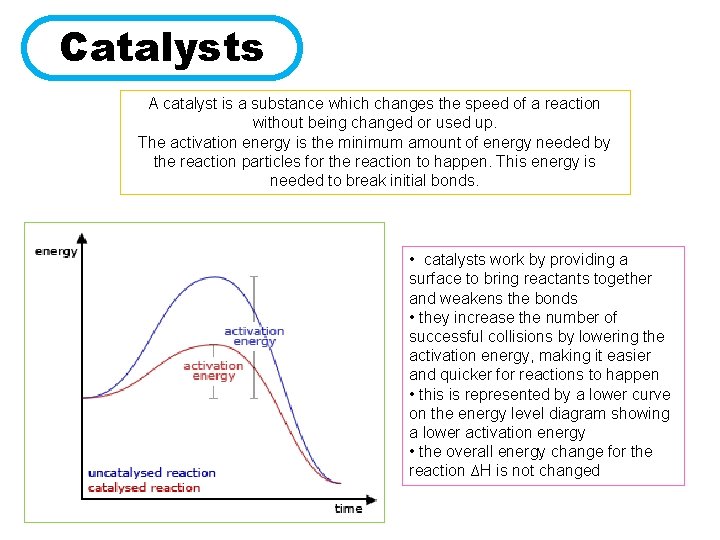
Catalysts A catalyst is a substance which changes the speed of a reaction without being changed or used up. The activation energy is the minimum amount of energy needed by the reaction particles for the reaction to happen. This energy is needed to break initial bonds. • catalysts work by providing a surface to bring reactants together and weakens the bonds • they increase the number of successful collisions by lowering the activation energy, making it easier and quicker for reactions to happen • this is represented by a lower curve on the energy level diagram showing a lower activation energy • the overall energy change for the reaction ∆H is not changed

Bond Energy Every chemical bond has a particular bond energy associated with it. This bond energy is slightly different depending what compound the bond occurs in. Total Energy Change = Total Energy For Breaking Bonds – Total Energy For Making Bonds Calculate the energy change for the reaction H 2 + Cl 2 → 2 HCl H-H: 436 k. J/mol Cl-Cl: 242 k. J/mol H-Cl: 431 k. J/mol 1) breaking H-H and Cl-Cl: 436+242= 678 k. J 2) making 2 H-Cl: 431 x 2= 862 k. J 3) 678 -862= -184 k. J/mol This is an exothermic reaction as the ∆H is negative.

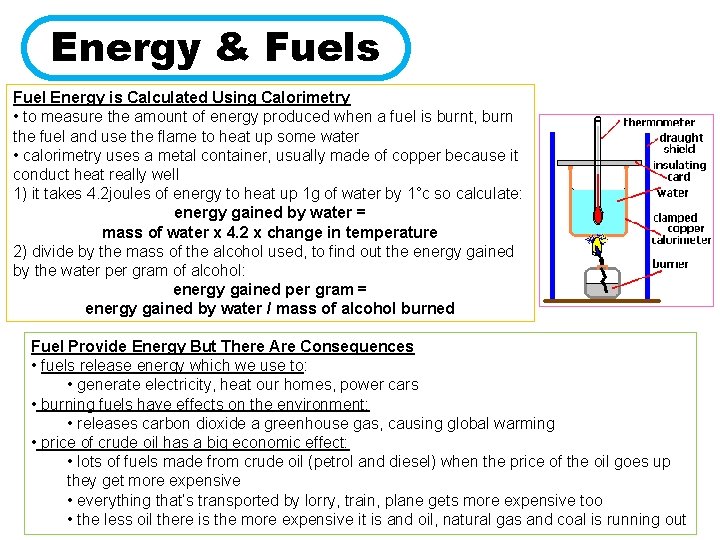
Energy & Fuels Fuel Energy is Calculated Using Calorimetry • to measure the amount of energy produced when a fuel is burnt, burn the fuel and use the flame to heat up some water • calorimetry uses a metal container, usually made of copper because it conduct heat really well 1) it takes 4. 2 joules of energy to heat up 1 g of water by 1°c so calculate: energy gained by water = mass of water x 4. 2 x change in temperature 2) divide by the mass of the alcohol used, to find out the energy gained by the water per gram of alcohol: energy gained per gram = energy gained by water / mass of alcohol burned Fuel Provide Energy But There Are Consequences • fuels release energy which we use to: • generate electricity, heat our homes, power cars • burning fuels have effects on the environment: • releases carbon dioxide a greenhouse gas, causing global warming • price of crude oil has a big economic effect: • lots of fuels made from crude oil (petrol and diesel) when the price of the oil goes up they get more expensive • everything that’s transported by lorry, train, plane gets more expensive too • the less oil there is the more expensive it is and oil, natural gas and coal is running out

Energy & Food Energy: Measured In Calories and Kilocalories • 1 calorie is the amount of energy needed to raise the temperature of 1 g of water by 1°c, so 1 c = 4. 2 J • 1 Calorie is the amount of energy needed to raise the temperature of 1 kg of water by 1°c, so 1 C or 1 kcal = 4200 J Our Energy Is From Food • different foods produce different amounts of energy • the composition/structure of the food determines how much energy it produces • foods with high portions of fats and oils produce large amounts of energy • carbohydrates produce some energy but much less than fats and oils • proteins contain about as much energy as carbohydrates but we don’t normally use it for energy in our bodies Taking In More Fuel Than You Use Means Excess Is Stored • our bodies needs energy to perform all our daily activities including tasks you do without thinking • energy in food is released by the respiration happening in our cells all the time: glucose + oxygen → carbon dioxide + water + energy • eating foods containing more energy than your body needs will cause the body to store excess food as fat, continually doing this will eventually make you obese • when the food you eat contains less energy than our body needs our body uses up some of its fat stores, so fat is gradually lost • calorie controlled diets are low fat diets and usually avoid sugar because it’s high in energy and it stimulates the appetite, they recommend slow release carbohydrates like whole meal bread as they fill you up for longer and making you less likely to eat snacks between meals

Test For Cations Flame Tests Identify Metal Ions • lithium Li+ burns with a crimson red flame • sodium Na+ burns with a yellow orange flame • potassium K+ burns with a lilac flame • calcium Ca 2+ burns with a brick red flame • barium Ba 2+ burns with a green flame To Test For Ammonium Ions NH 4+ • add sodium hydroxide (Na. OH) to the unknown substance • ammonium compound + sodium hydroxide gives of ammonia • ammonia smells so you can tell if it is given off and it turns damp red litmus paper blue Some Metals Form A Coloured Precipitate with Na. OH • add a few drops of sodium hydroxide solution to the solution of the unknown compound • if you get a coloured insoluble hydroxide you can tell which metal was in the compound • calcium: Ca+ + 2 OH- → Ca(OH)2 white • copper II: Cu 2+ + 2 OH- → Cu(OH)2 blue • iron II: Fe 2+ + 2 OH- → Fe(OH)2 sludgy green • iron III: Fe 3+ + 3 OH- → Fe(OH)3 reddish brown • aluminum: Al 3+ + 3 OH- → Al(OH)3 white • then redissolves in excess Na. OH: Al(OH)3 + OH- → Al(OH)4 - colorless solution • magnesium: Mg 2+ + 2 OH- → Mg(OH)2 white

Test For Anions Test For Carbonates carbonate + acid → salt + water + carbon dioxide • add dilute acid (hydrochloric acid) to see if it will form CO 2 • to test if the gas is CO 2 bubble it through limewater and if it is it will turn from clear to milky/cloudy Some carbonates change colour when they decompose • copper carbonate – green to black, stays black Cu. CO 3 (s, green) → Cu. O (s, black) + CO 2 (g) • zinc carbonate – white to yellow, cools turns back to white Zn. CO 3 (s, white) → Zn. O (s, yellow-hot, white-cold) + CO 2 (g) Test For Halides • add dilute nitric acid to the halide solution then silver nitrate solution • chloride: white precipitates of silver chloride Ag+ (aq) + Cl- (aq) → Ag. Cl (s) • bromide: cream precipitates of silver bromide Ag+ (aq) + Br- (aq) → Br. Cl (s) • iodide: yellow precipitates of silver iodide Ag+ (aq) + I- (aq) → ICl (s) Test For Nitrates NO 3 • add aluminium power then sodium hydroxide solution and heat • it should be reduced to ammonia which can be smelt and will turn damp red litmus paper blue Test For Sulphates – Sulphate Ion SO 42 • add dilute hydrochloric acid to the sulphate ion solution then barium chloride solution • a white precipitate of barium sulphate should form • Ba 2+ (aq) + SO 42 - (aq) → Ba. SO 4 (S)

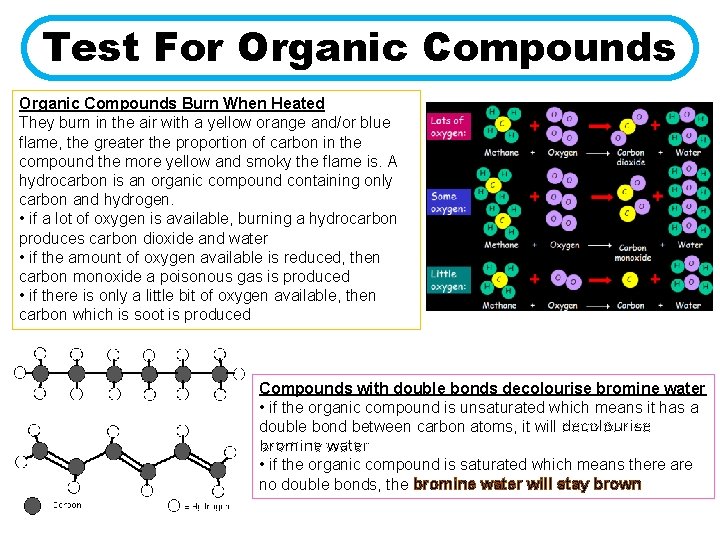
Test For Organic Compounds Burn When Heated They burn in the air with a yellow orange and/or blue flame, the greater the proportion of carbon in the compound the more yellow and smoky the flame is. A hydrocarbon is an organic compound containing only carbon and hydrogen. • if a lot of oxygen is available, burning a hydrocarbon produces carbon dioxide and water • if the amount of oxygen available is reduced, then carbon monoxide a poisonous gas is produced • if there is only a little bit of oxygen available, then carbon which is soot is produced Compounds with double bonds decolourise bromine water • if the organic compound is unsaturated which means it has a double bond between carbon atoms, it will decolourise bromine water • if the organic compound is saturated which means there are no double bonds, the bromine water will stay brown

Empirical Formula to find the empirical formula of an organic compound by burning it An empirical formula shows the ratios of all the elements in a substance. We can work out the empirical formula of an organic compound by burning a known mass of it completely in oxygen and then measuring the masses of all the products. Hydrocarbon + Oxygen → Carbon Dioxide + Water A hydrocarbon is an organic compound containing only carbon and hydrogen. Finding the Empirical Formula 1) find mass of each element: (mass of compound) x (relative atomic mass/relative formula mass) carbon in CO 2: (mass of CO 2) x (12/44) hydrogen: (mass of H 2 O) x (2/18) 2) find the number of moles of carbon and hydrogen: (mass of element) / (atomic mass) carbon: (mass of C) / (12) hydrogen: (mass of H) / (1) 3) divide both answers by the smallest answer to give the simplest ration. 4) Write down the empirical formula. 0. 4 g of an organic hydrocarbon is burn completely in oxygen. 1. 1 g of carbon dioxide and 0. 9 g of water are formed. What is the compound’s empirical formula? 1) C: 1. 1 x (12/44) = 0. 3 g H: 0. 9 x (2/18) = 0. 1 g 2) C: 0. 3 g / 12 = 0. 025 mol H: 0. 1 g / 1 = 0. 1 mol 3) C: 0. 025 / 0. 025 = 1 H: 0. 1 / 0. 025 = 4 4) CH 4

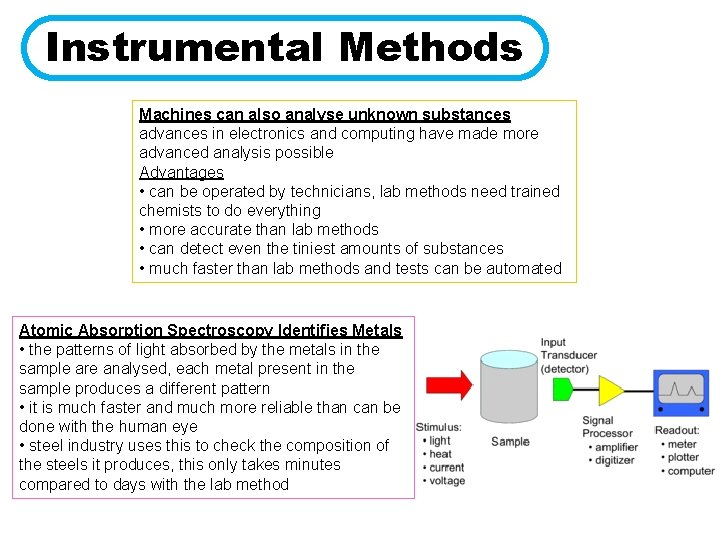
Instrumental Methods Machines can also analyse unknown substances advances in electronics and computing have made more advanced analysis possible Advantages • can be operated by technicians, lab methods need trained chemists to do everything • more accurate than lab methods • can detect even the tiniest amounts of substances • much faster than lab methods and tests can be automated Atomic Absorption Spectroscopy Identifies Metals • the patterns of light absorbed by the metals in the sample are analysed, each metal present in the sample produces a different pattern • it is much faster and much more reliable than can be done with the human eye • steel industry uses this to check the composition of the steels it produces, this only takes minutes compared to days with the lab method

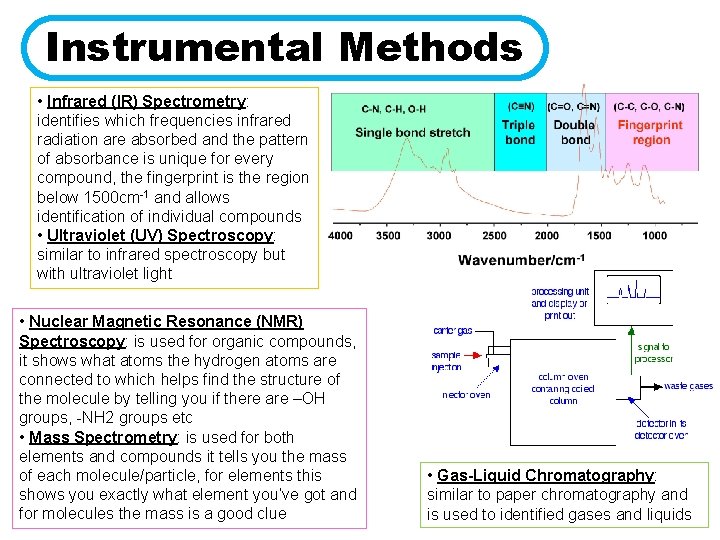
Instrumental Methods • Infrared (IR) Spectrometry: identifies which frequencies infrared radiation are absorbed and the pattern of absorbance is unique for every compound, the fingerprint is the region below 1500 cm-1 and allows identification of individual compounds • Ultraviolet (UV) Spectroscopy: similar to infrared spectroscopy but with ultraviolet light • Nuclear Magnetic Resonance (NMR) Spectroscopy: is used for organic compounds, it shows what atoms the hydrogen atoms are connected to which helps find the structure of the molecule by telling you if there are –OH groups, -NH 2 groups etc • Mass Spectrometry: is used for both elements and compounds it tells you the mass of each molecule/particle, for elements this shows you exactly what element you’ve got and for molecules the mass is a good clue • Gas-Liquid Chromatography: similar to paper chromatography and is used to identified gases and liquids