Chemistry 20 Review Predicting Polarity Steps 1 Draw








- Slides: 10

Chemistry 20

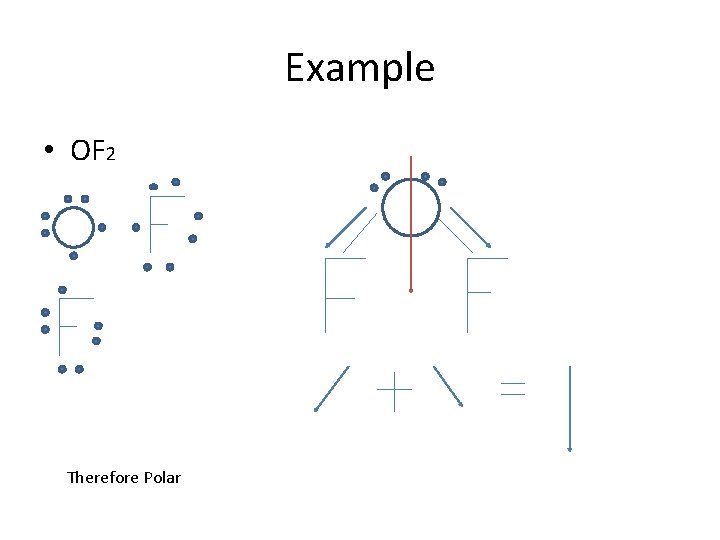
Review • Predicting Polarity – Steps 1: Draw the Lewis Formula – Step 2: Bases on VSEPR Theory, draw the stereochemical formula – Step 3: Add the electronegativities, assign δ + or δ – Step 4: Draw in the bond dipoles – Step 5: Add the vectors – If cancel each other out or add to zero – non polar – If they do not cancel each other out – polar

Example • OF 2 Therefore Polar

• Dipole – attractions between dipoles • London Forces- due to the simultaneous attraction between a momentary dipole in a molecule and the momentary dipoles in the surrounding molecules

Predicting with Dipole – Dipole and London Forces • If other factors are equal then: – The more polar the molecule, the strong the dipole – dipole force therefore the higher boiling point it will have – The greater the number of electrons per molecule, the stronger the London force and therefore the higher boiling point

• You can explain and predict the relative boiling points of two substances if – The London force is the same but the dipole force is different – The dipole-dipole force is the same but the London force is different – Both the London force and the Dipole- Dipole force are greater for one substance

You cannot explain and predict the relative boiling points of two substances if • One of the substances has a stronger dipole force, and the other substance has a stronger London force • The molecules of the two substances differ significantly in shape • The central atom of either molecule has an incomplete octet

Example Problem • Using London Forces and dipole-dipole forces, state the kind of intermolecular forces (s) present between molecules of the following substance – Water (Solvent) - London forces – Carbon Dioxide (Dry Ice) – London Forces – Methane – London Forces

Questions • Page 109 – Questions 2 & 3

Hydrogen Bonding • Hydrogen nucleus could be shared between pairs of electrons on adjacent molecules