CHEMISTRY 20 CHEMICAL BONDING Polarity Morning Assignment Review

CHEMISTRY 20 CHEMICAL BONDING Polarity
Morning Assignment Review valence levels and periodic table Bohr model: periodic table shaped based on filling of e- levels Eg. 2, 8, 8, 18, 32+ � Group # - tells how many valence e- (esp gr 1 -2, 13 -18) � Period # - tells how many energy levels are occupied Quantum Mechanics – orbitals can contain 0, 1, or 2 e� Bonding Above theory – energy level 1 has room for 1 orbital. level 1 there are room for 4 orbitals (8 e-) Called octet rule. Usually obeyed by Gr. 1, 2 13 -18 (C, N, O,
Polarity of Molecules are said to be polar if they have a pos. & neg. end. � How does this happen? � Molecular Dipoles – unequal electron sharing Polar molecule – molecule which has an overall charge separation. (pos. end & neg. end) Non-polar molecule – no net charge separation � Neutral in charge
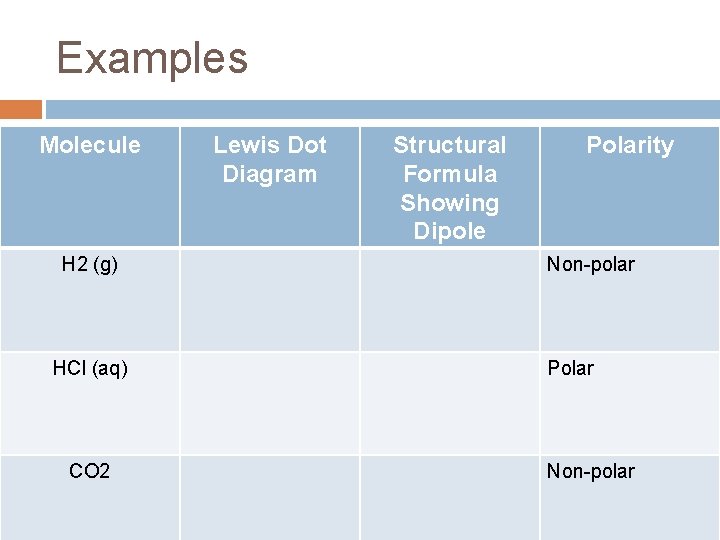
Examples Molecule H 2 (g) HCl (aq) CO 2 Lewis Dot Diagram Structural Formula Showing Dipole Polarity Non-polar Polar Non-polar
Factors Affecting Molecular Dipoles Electronegativity of atoms – more electronegativity = more e- pull Bond Dipoles – has to do with electronegativities and electron shifting as well Shape of Molecules (Stereochemical formula)
Determining Polarity of Molecules Need to know � Stereochemistry � Bond dipoles (molecule shape)
Steps to Determine Polarity 1. 2. 3. 4. Draw Lewis Dot Diagram Apply VSEPR rule to draw shape of the molecule Use electronegativities to determine bond dipoles Use the above info to determine whether the molecule is polar or non-polar
Example: H 2 O (l)
Bonding Continuum Draw bonding continuum from notes The different bonds are just the extremes on the continuum � It’s a different degree rather than a different kind

Assignment Reading � Text Pages 98 – 104 Questions � SNAP Page 69
- Slides: 10