CHEMISTRY 2 Honors Unit 5 Organic Chemistry and







- Slides: 19

CHEMISTRY 2 Honors Unit 5 Organic Chemistry and Biochemistry Jeff Venables Northwestern High School

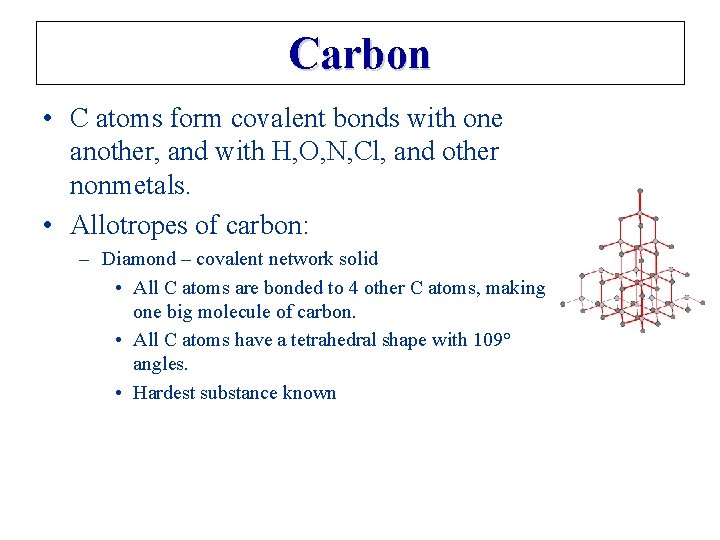
Carbon • C atoms form covalent bonds with one another, and with H, O, N, Cl, and other nonmetals. • Allotropes of carbon: – Diamond – covalent network solid • All C atoms are bonded to 4 other C atoms, making one big molecule of carbon. • All C atoms have a tetrahedral shape with 109° angles. • Hardest substance known

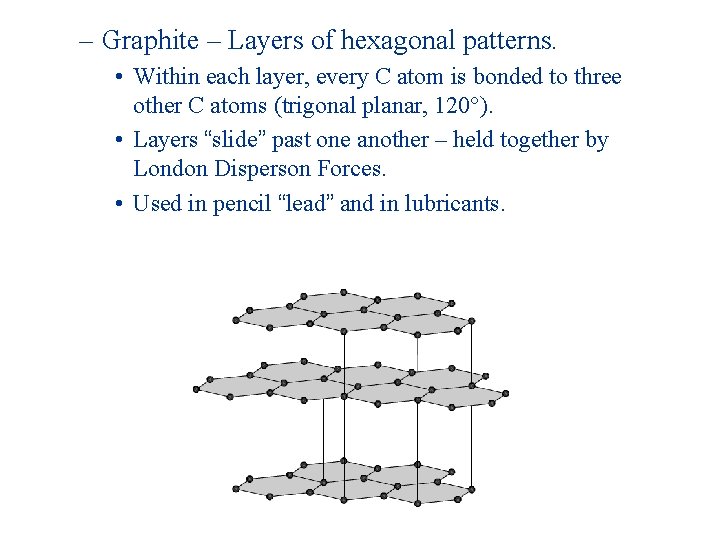
– Graphite – Layers of hexagonal patterns. • Within each layer, every C atom is bonded to three other C atoms (trigonal planar, 120°). • Layers “slide” past one another – held together by London Disperson Forces. • Used in pencil “lead” and in lubricants.

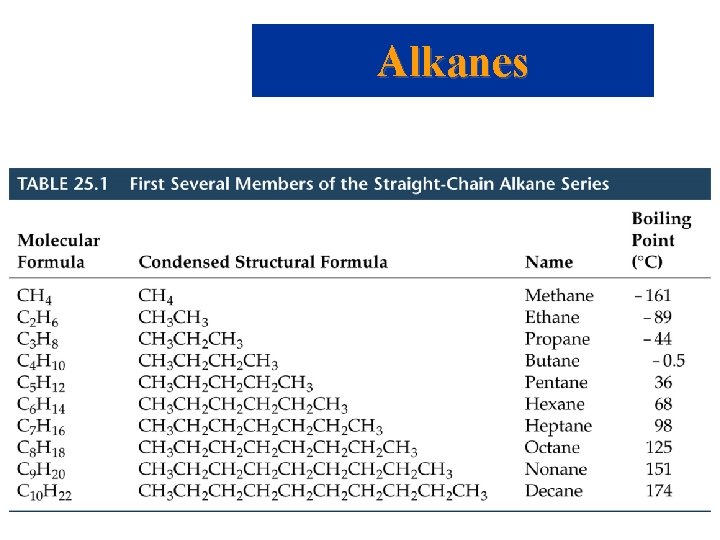
Organic Compounds • Hydrocarbons – composed only of hydrogen and carbon. • All Carbon atoms have 4 bonds, hydrogen and halogen atoms have 1 bond, oxygen 2 bonds, and nitrogen 3 bonds. – Alkanes – also called saturated hydrocarbons • Formula is Cn. H 2 n+2 • Named based on number of carbon atoms: 1 = methane 2 = ethane 3 = propane 4 = butane 5 = pentane 6 = hexane 7 = heptane 8 = octane 9 = nonane 10 = decane

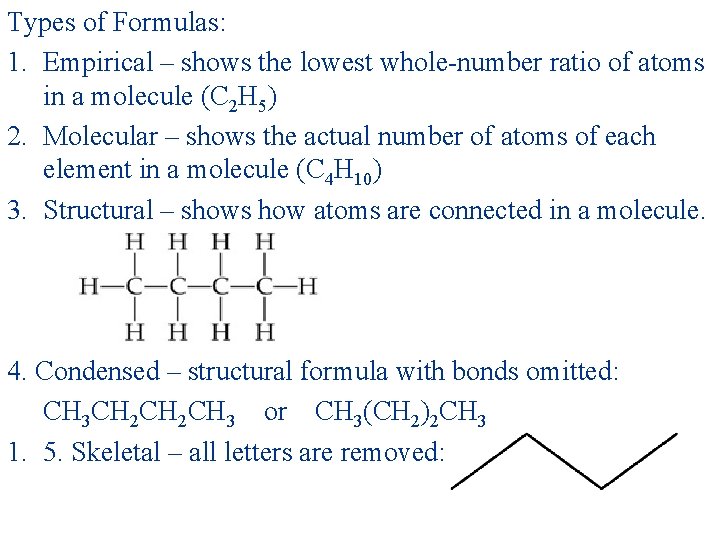
Types of Formulas: 1. Empirical – shows the lowest whole-number ratio of atoms in a molecule (C 2 H 5) 2. Molecular – shows the actual number of atoms of each element in a molecule (C 4 H 10) 3. Structural – shows how atoms are connected in a molecule. 4. Condensed – structural formula with bonds omitted: CH 3 CH 2 CH 3 or CH 3(CH 2)2 CH 3 1. 5. Skeletal – all letters are removed:

Alkanes • The name of alkanes varies according to the number of C atoms present in the chain. • Since the only intermolecular forces available to alkanes are London dispersion forces, the boiling points increase smoothly as the molar mass increases. • Methane to butane are gases at normal pressures. • Pentane to decane are liquids at normal pressures. • Each carbon in an alkane has 4 single bonds.

Alkanes

Alkanes • Names based on number of carbon atoms: 1 = meth 6 = hex 2 = eth 7 = hept 3 = prop 8 = oct 4 = but 9 = non 5 = pent 10 = dec • All end with -ane

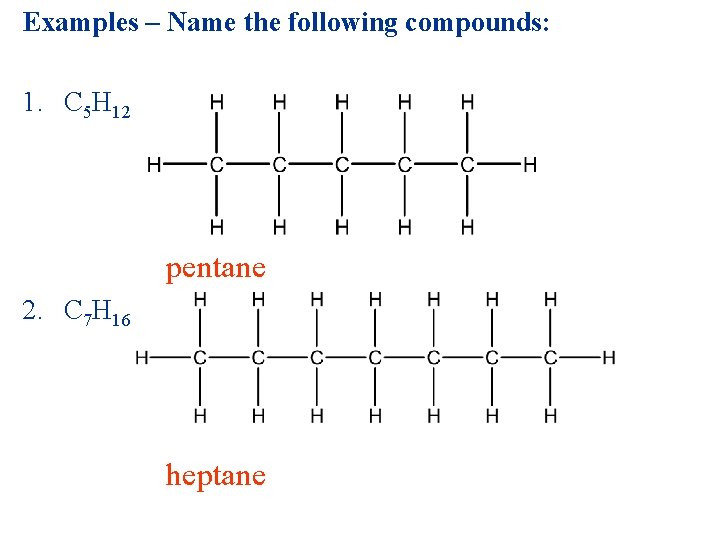
Examples – Name the following compounds: 1. C 5 H 12 pentane 2. C 7 H 16 heptane

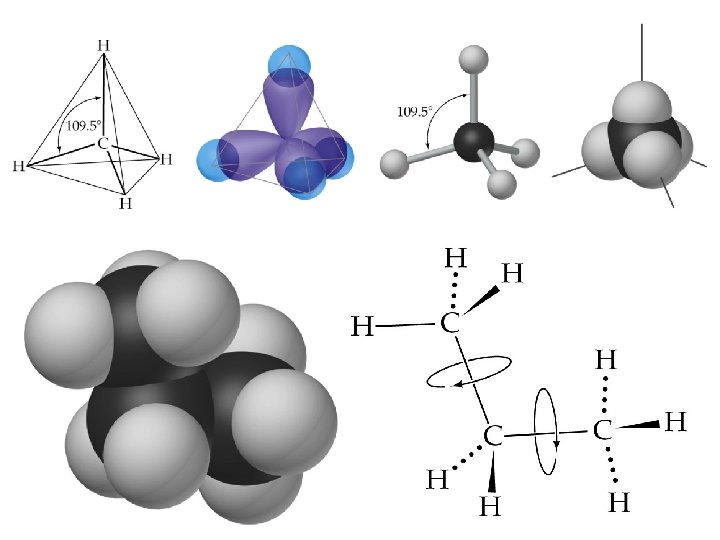
Alkanes Structures of Alkanes • VSEPR theory predicts each C atom is tetrahedral. • It is easy to rotate about the C-C bond in alkanes.


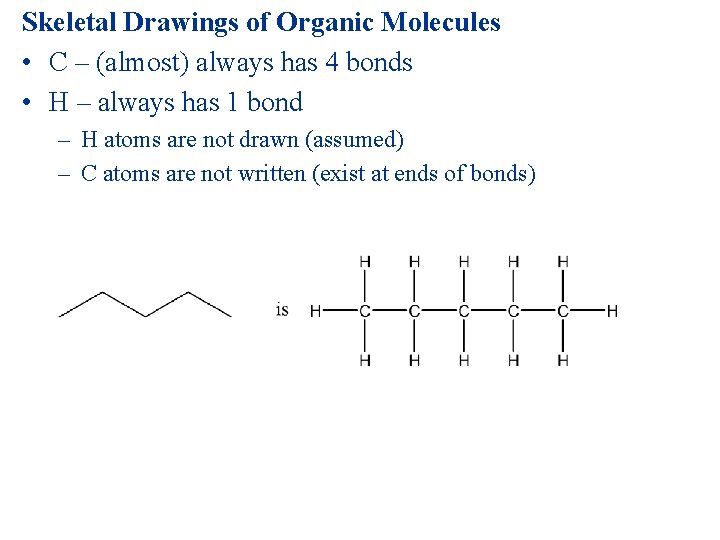
Skeletal Drawings of Organic Molecules • C – (almost) always has 4 bonds • H – always has 1 bond – H atoms are not drawn (assumed) – C atoms are not written (exist at ends of bonds)

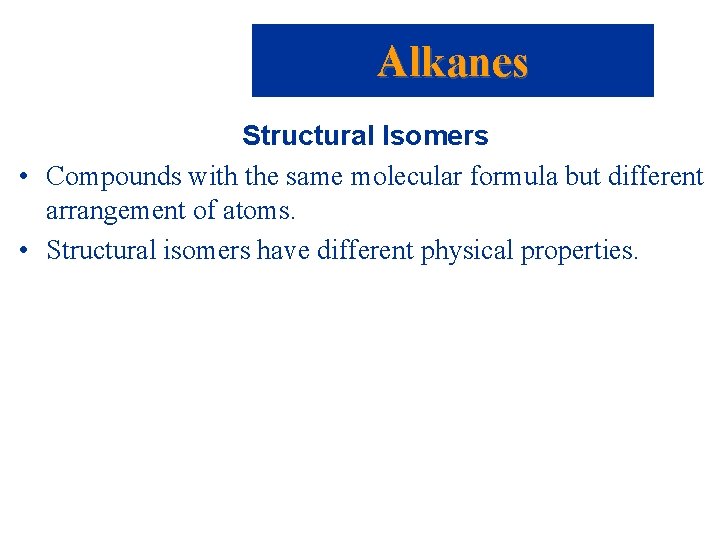
Alkanes Structural Isomers • Compounds with the same molecular formula but different arrangement of atoms. • Structural isomers have different physical properties.


Alkanes Nomenclature of Alkanes • All organic molecule names have three parts: − Prefix, which tells the nature of the substituents; − Base, which gives the number of carbons; and the − Suffix, which gives the family (alkanes, etc. ). • Rules for naming compounds are given by the International Union for Pure and Applied Chemistry (IUPAC).

• To name alkanes: – Find the longest continuous carbon chain and use it as the name of the compound. – Number the carbon atoms starting with the end closest to the substituent. – Name and give the location of each substituent. • When two or more substituents are present list them in alphabetical order.

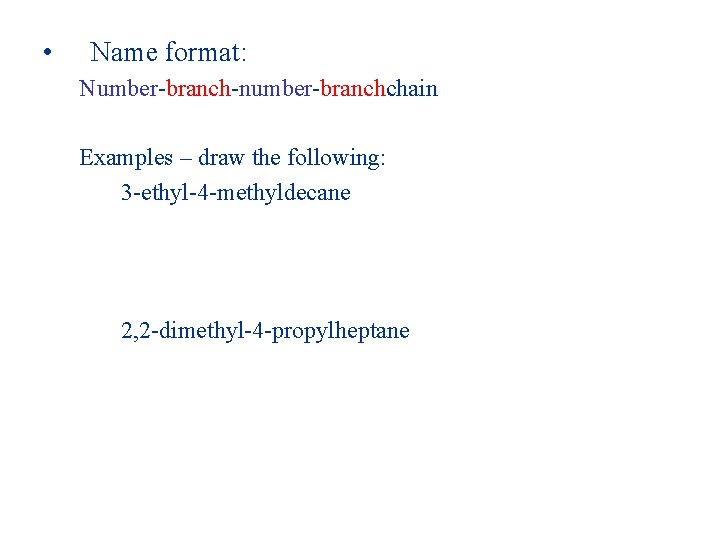
• Name format: Number-branch-number-branchchain Examples – draw the following: 3 -ethyl-4 -methyldecane 2, 2 -dimethyl-4 -propylheptane

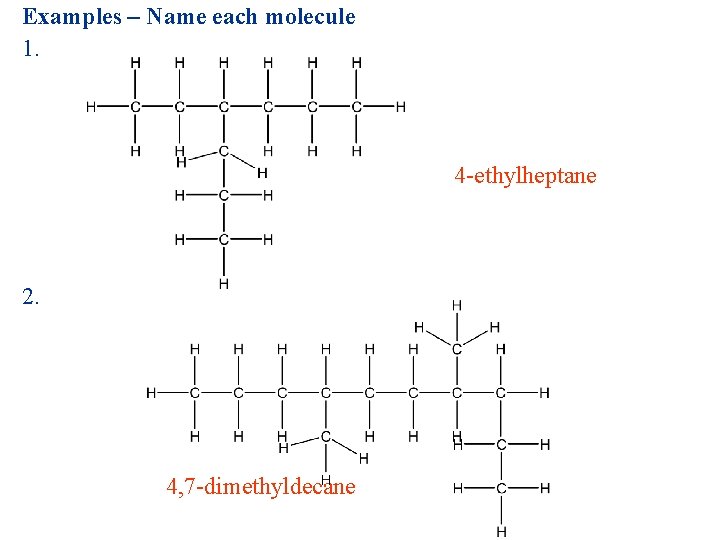
Examples – Name each molecule 1. 4 -ethylheptane 2. 4, 7 -dimethyldecane

3. 5 -ethyl-3 -methyloctane 4. 3 -ethyl-4, 7 -dimethyloctane