Chemistry 2 2 Identify Ions AS 91162 Ionic
- Slides: 4

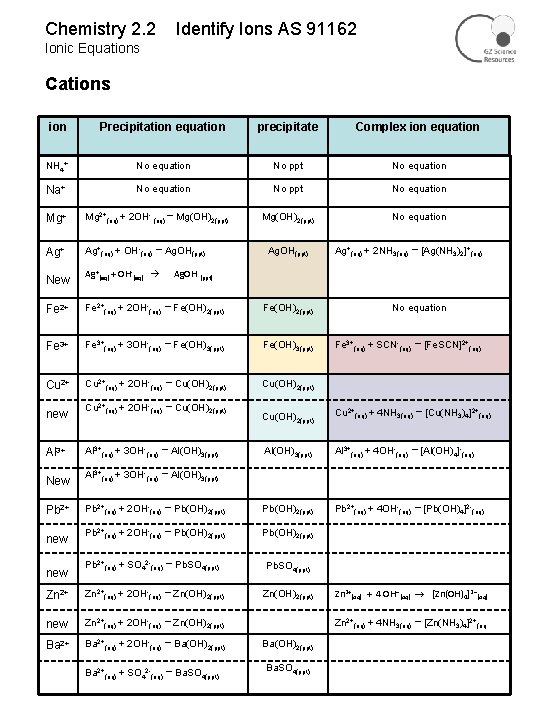
Chemistry 2. 2 Identify Ions AS 91162 Ionic Equations Cations ion Precipitation equation precipitate Complex ion equation NH 4+ No equation No ppt No equation Na+ No equation No ppt No equation Mg(OH)2(ppt) No equation Mg+ Mg 2+(aq) + 2 OH- (aq) → Mg(OH)2(ppt) Ag+(aq) + OH-(aq) New → Ag. OH(ppt) Ag+(aq) + OH-(aq) → Ag. OH(ppt) Ag+(aq) + 2 NH 3(aq) → [Ag(NH 3)2]+(aq) Ag. OH (ppt) Fe 2+(aq) + 2 OH-(aq) → Fe(OH)2(ppt) Fe 3+(aq) + 3 OH-(aq) → Fe(OH)3(ppt) Cu 2+(aq) + 2 OH-(aq) → Cu(OH)2(ppt) new Cu 2+(aq) + 2 OH-(aq) → Cu(OH)2(ppt) Al 3+(aq) + 3 OH-(aq) → Al(OH)3(ppt) New Al 3+(aq) + 3 OH-(aq) → Al(OH)3(ppt) Pb 2+(aq) + 2 OH-(aq) → new Pb 2+(aq) + 2 OH-(aq) No equation Fe 3+(aq) + SCN-(aq) → [Fe. SCN]2+(aq) Cu(OH)2(ppt) Cu 2+(aq) + 4 NH 3(aq) → [Cu(NH 3)4]2+(aq) Al(OH)3(ppt) Al 3+(aq) + 4 OH-(aq) Pb(OH)2(ppt) Pb 2+(aq) + 4 OH-(aq) → Pb(OH)2(ppt) Pb 2+(aq) + SO 42 -(aq) → Pb. SO 4(ppt) Zn 2+(aq) + 2 OH-(aq) → Zn(OH)2(ppt) new Zn 2+(aq) + 2 OH-(aq) → Zn(OH)2(ppt) Ba 2+(aq) + 2 OH-(aq) → Ba(OH)2(ppt) Ba 2+(aq) + SO 42 -(aq) → Ba. SO 4(ppt) new → [Al(OH)4]-(aq) → [Pb(OH)4]2 -(aq) Zn 2+(aq) + 4 OH (aq) [Zn(OH)4]2 (aq) Zn 2+(aq) + 4 NH 3(aq) → [Zn(NH 3)4]2+(aq

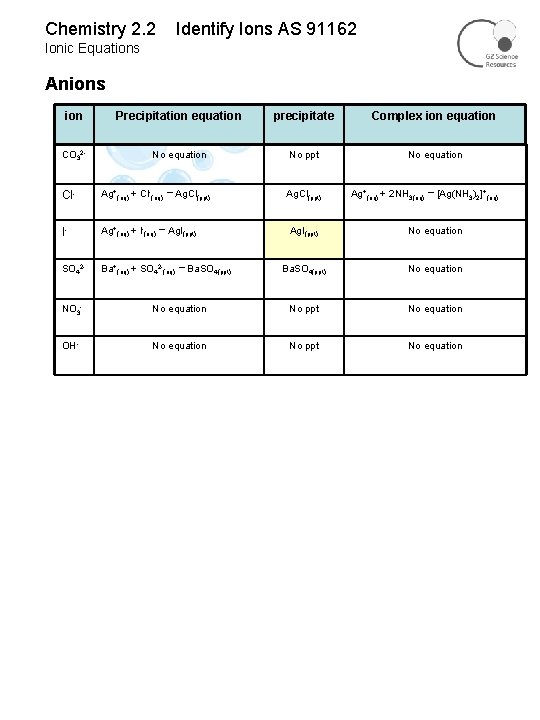
Chemistry 2. 2 Identify Ions AS 91162 Ionic Equations Anions ion Precipitation equation precipitate Complex ion equation CO 32 - No equation No ppt No equation Cl- Ag+(aq) + Cl-(aq) → I- Ag+(aq) + l-(aq) Ag. I(ppt) SO 42 - Ba+(aq) + SO 42 -(aq) → Ag. Cl(ppt) → Ba. SO 4(ppt) Ag. Cl(ppt) Ag+(aq) + 2 NH 3(aq) → [Ag(NH 3)2]+(aq) Ag. I(ppt) No equation Ba. SO 4(ppt) No equation NO 3 - No equation No ppt No equation OH- No equation No ppt No equation

Cations ion NH 4+ Na+ Mg+ Ag+ Fe 2+ Fe 3+ Cu 2+ Al 3+ Pb 2+ Zn 2+ Ba 2+ Precipitation equation precipitate Complex ion equation

Anions ion CO 32 - Cl. ISO 42 - NO 3 OH- Precipitation equation precipitate Complex ion equation