CHEMISTRY 161 Chapter 9 Periodic Table of the

CHEMISTRY 161 Chapter 9
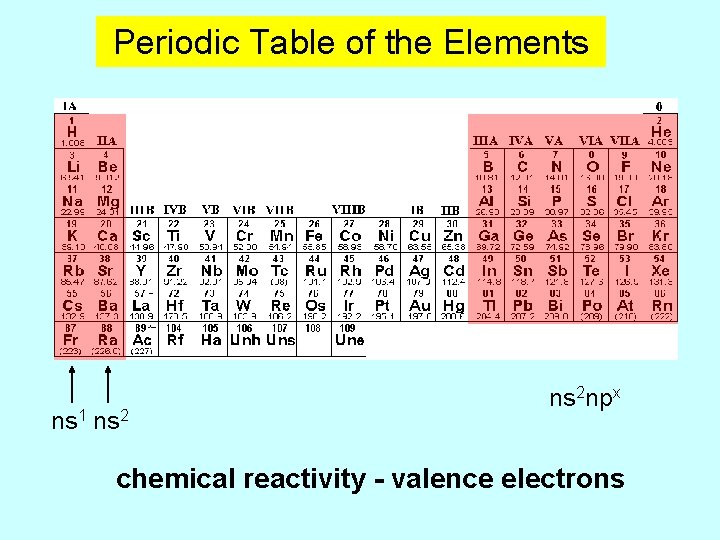
Periodic Table of the Elements ns 1 ns 2 npx chemical reactivity - valence electrons
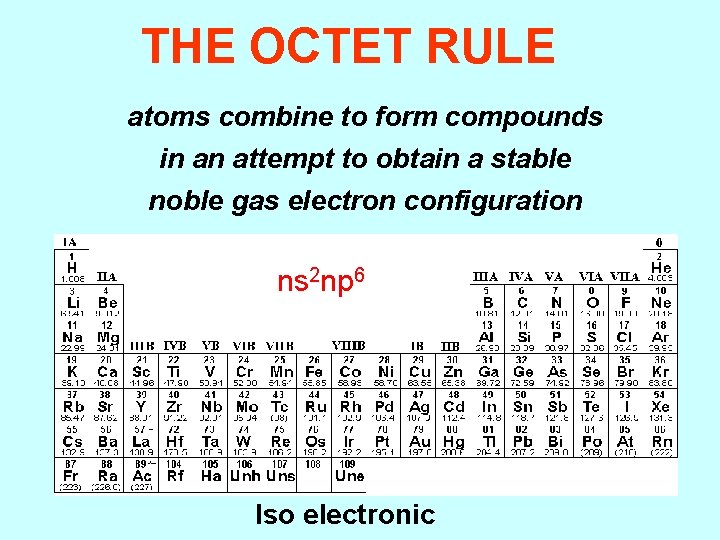
THE OCTET RULE atoms combine to form compounds in an attempt to obtain a stable noble gas electron configuration ns 2 np 6 Iso electronic
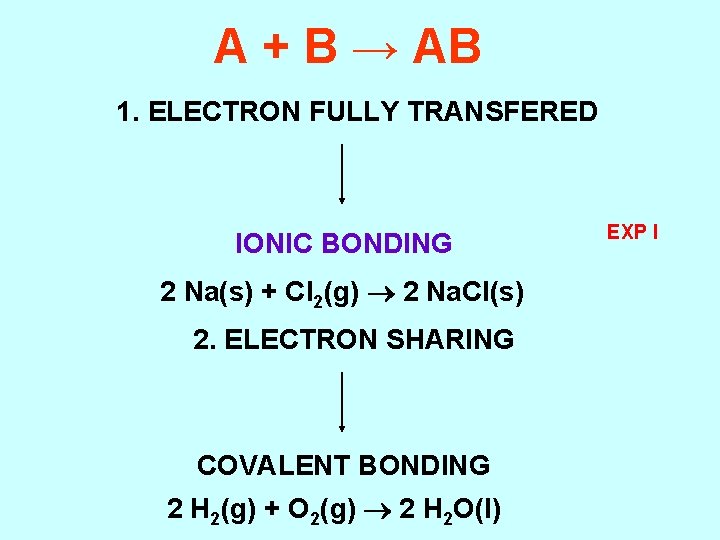
A + B → AB 1. ELECTRON FULLY TRANSFERED IONIC BONDING 2 Na(s) + Cl 2(g) 2 Na. Cl(s) 2. ELECTRON SHARING COVALENT BONDING 2 H 2(g) + O 2(g) 2 H 2 O(l) EXP I
LEWIS MODEL OF BONDING LEWIS DOT SYMBOL DOT represents one valence electron Gilbert Lewis (1875 -1946) . H
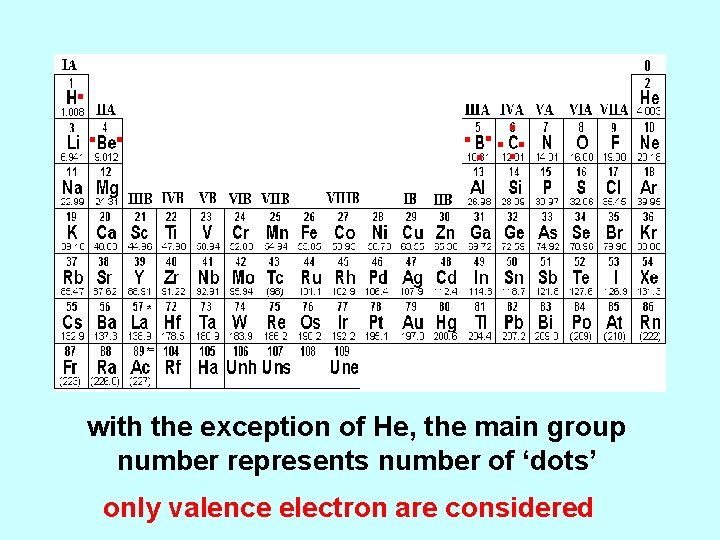
. . with the exception of He, the main group number represents number of ‘dots’ only valence electron are considered
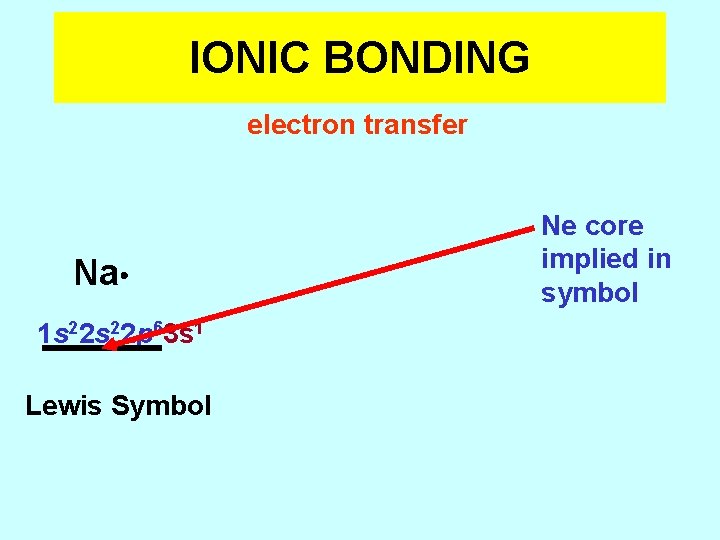
IONIC BONDING electron transfer Na 1 s 22 p 63 s 1 Lewis Symbol Ne core implied in symbol
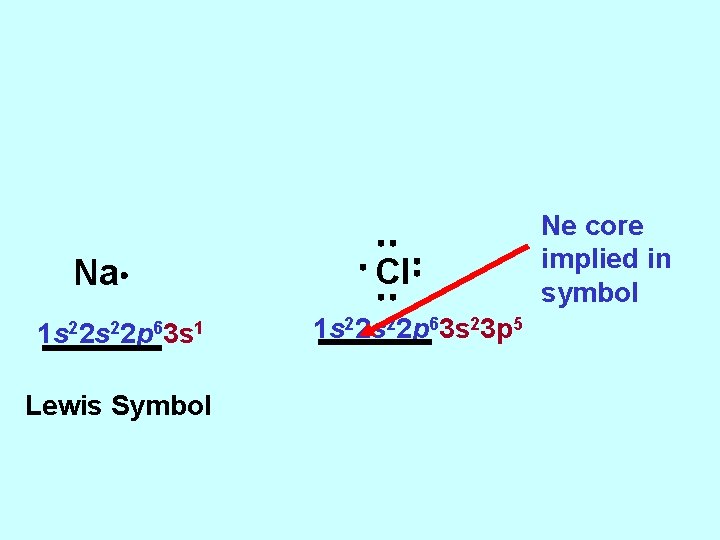
Na 1 s 22 p 63 s 1 Lewis Symbol Cl 1 s 22 p 63 s 23 p 5 Ne core implied in symbol
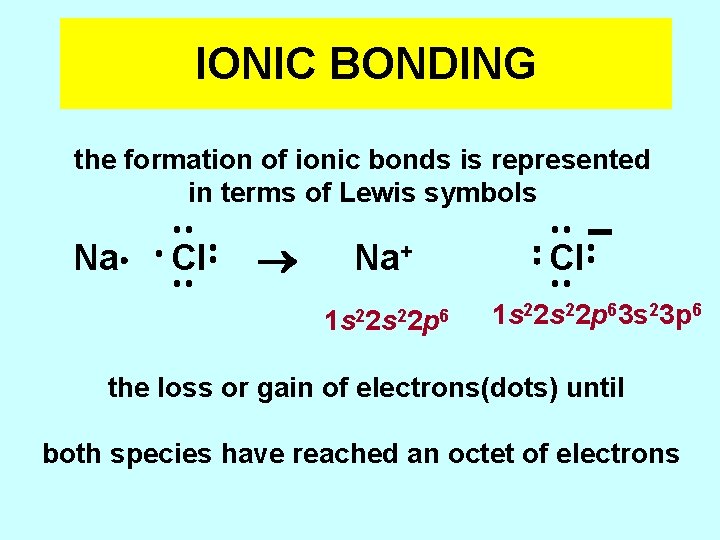
IONIC BONDING the formation of ionic bonds is represented in terms of Lewis symbols Na Cl Na+ 1 s 22 p 6 Cl 1 s 22 p 63 s 23 p 6 the loss or gain of electrons(dots) until both species have reached an octet of electrons
![Cl Cl [Ne] 3 s 23 p 6 represents one orbital (Pauli: 2 electrons) Cl Cl [Ne] 3 s 23 p 6 represents one orbital (Pauli: 2 electrons)](http://slidetodoc.com/presentation_image_h/c377f26ec70693d6374062f2a133393d/image-10.jpg)
Cl Cl [Ne] 3 s 23 p 6 represents one orbital (Pauli: 2 electrons)
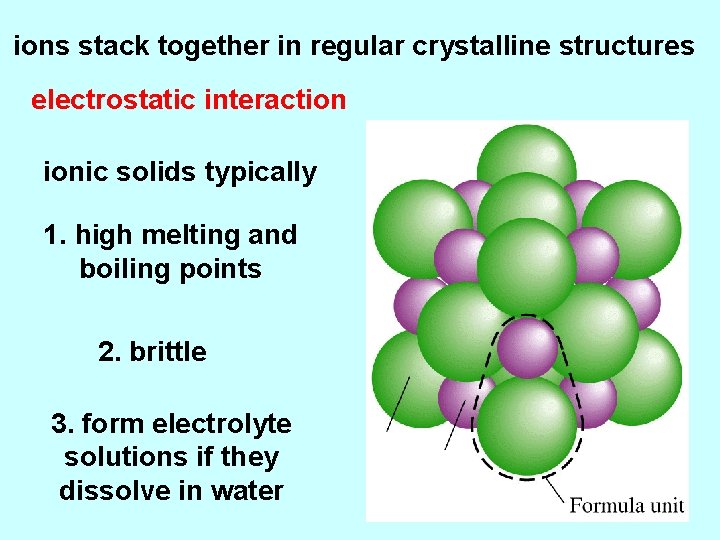
ions stack together in regular crystalline structures electrostatic interaction ionic solids typically 1. high melting and boiling points 2. brittle 3. form electrolyte solutions if they dissolve in water
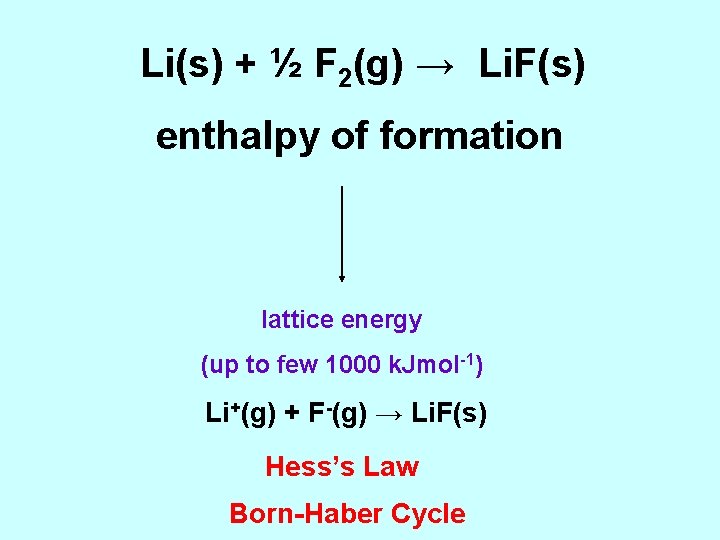
Li(s) + ½ F 2(g) → Li. F(s) enthalpy of formation lattice energy (up to few 1000 k. Jmol-1) Li+(g) + F-(g) → Li. F(s) Hess’s Law Born-Haber Cycle
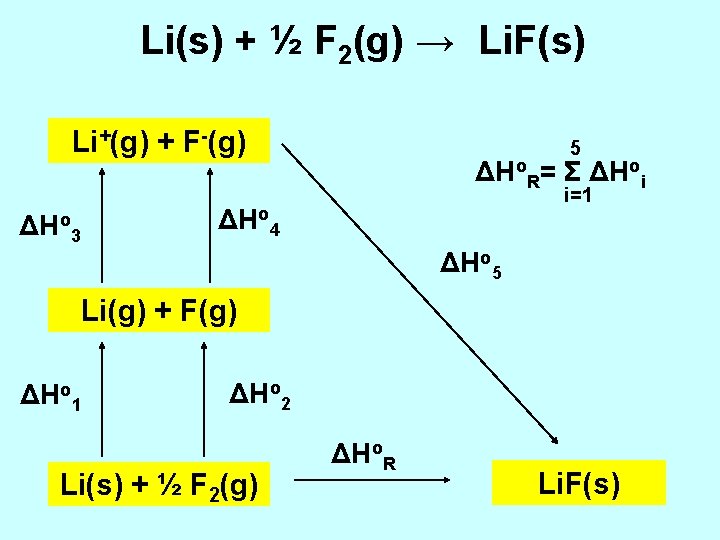
Li(s) + ½ F 2(g) → Li. F(s) Li+(g) + F-(g) ΔHo 3 5 ΔHo. R= Σ ΔHoi i=1 ΔHo 4 ΔHo 5 Li(g) + F(g) ΔHo 1 ΔHo 2 Li(s) + ½ F 2(g) ΔHo. R Li. F(s)
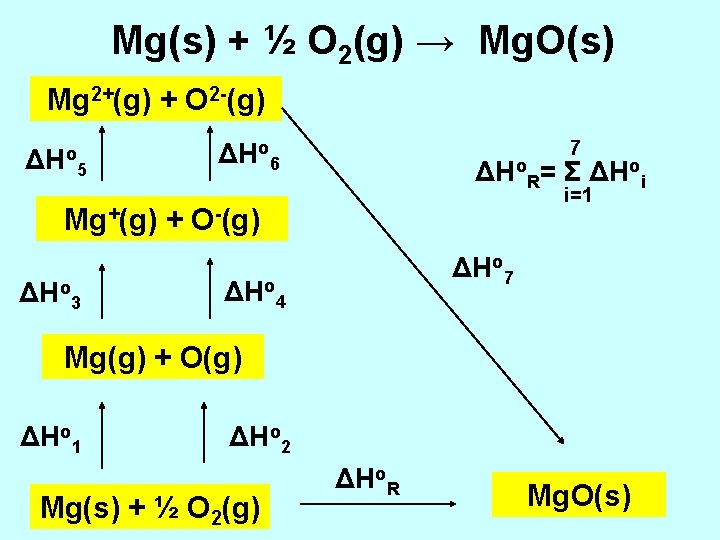
Mg(s) + ½ O 2(g) → Mg. O(s) Mg 2+(g) + O 2 -(g) ΔHo 5 7 ΔHo 6 ΔHo. R= Σ ΔHoi i=1 Mg+(g) + O-(g) ΔHo 3 ΔHo 7 ΔHo 4 Mg(g) + O(g) ΔHo 1 ΔHo 2 Mg(s) + ½ O 2(g) ΔHo. R Mg. O(s)
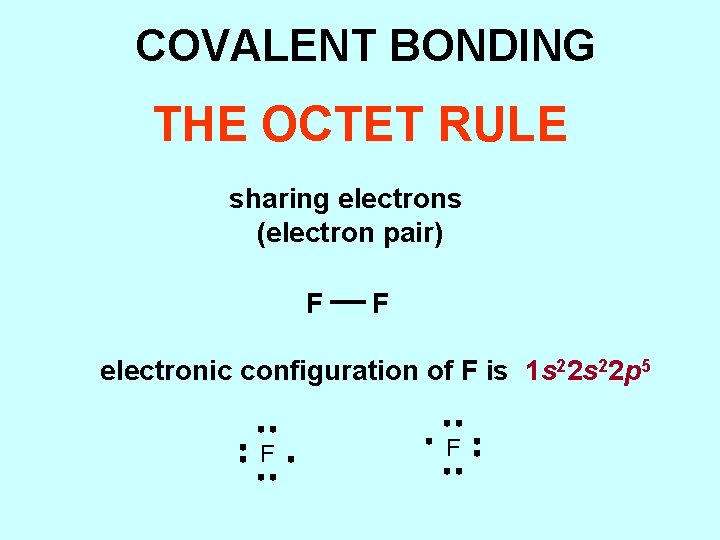
COVALENT BONDING THE OCTET RULE sharing electrons (electron pair) F F electronic configuration of F is 1 s 22 p 5 F F
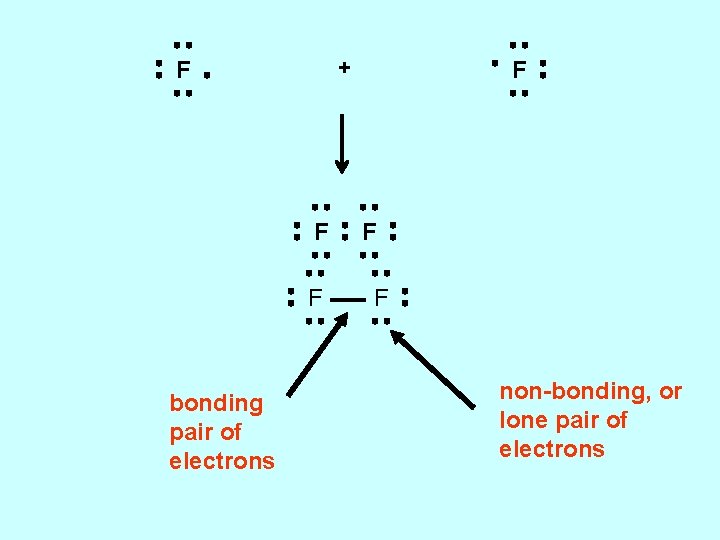
+ F F F bonding pair of electrons F F F non-bonding, or lone pair of electrons
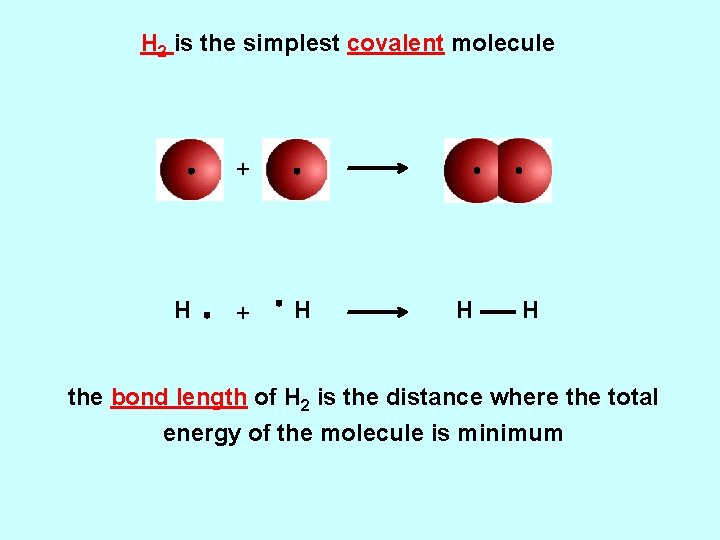
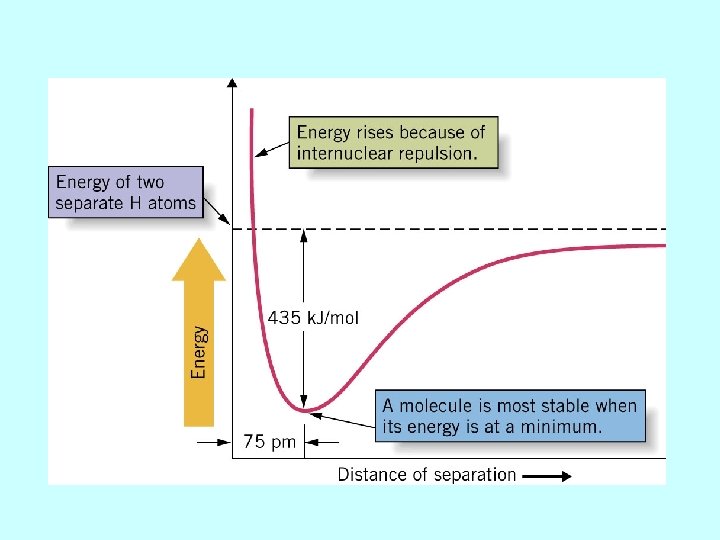
H 2 is the simplest covalent molecule + H H H the bond length of H 2 is the distance where the total energy of the molecule is minimum
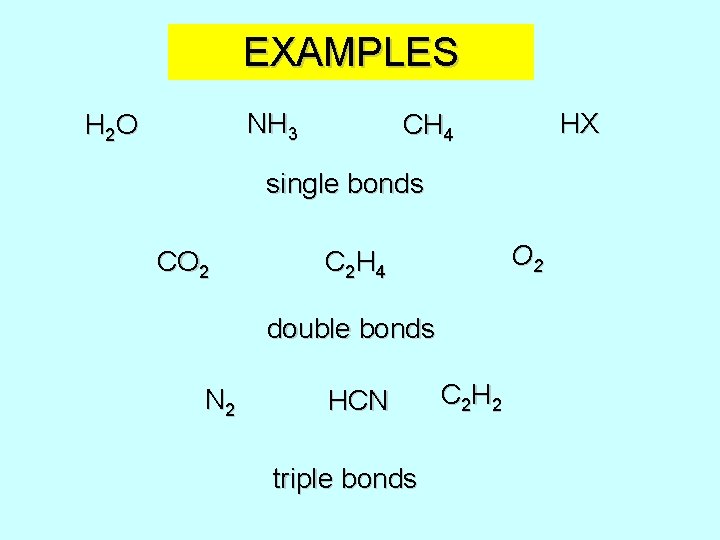
EXAMPLES NH 3 H 2 O HX CH 4 single bonds CO 2 C 2 H 4 double bonds N 2 HCN triple bonds C 2 H 2
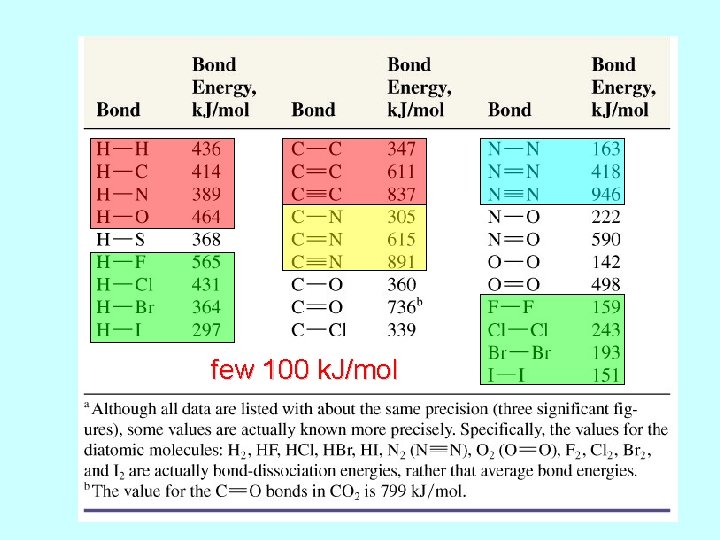
few 100 k. J/mol
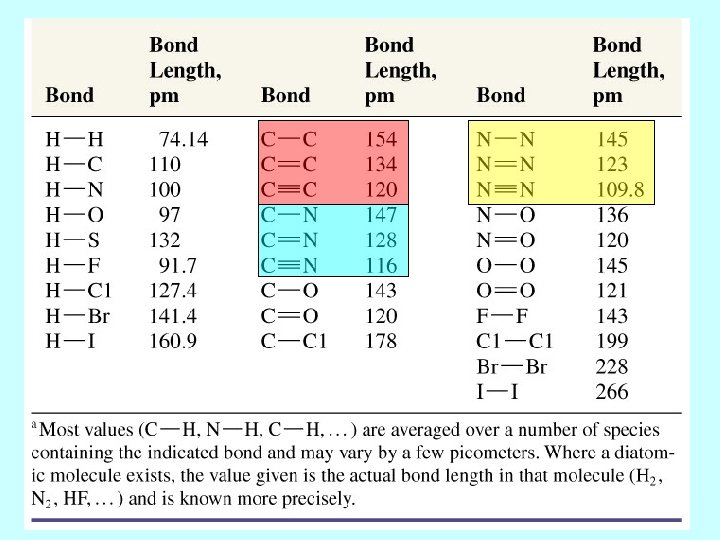
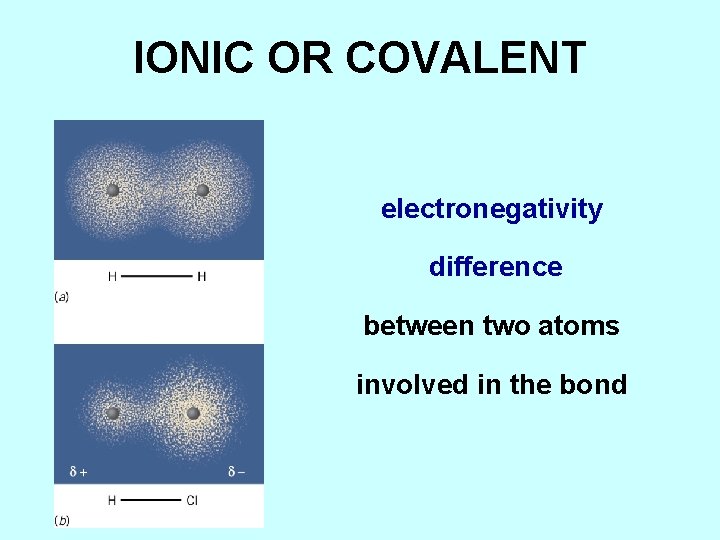
IONIC OR COVALENT electronegativity difference between two atoms involved in the bond
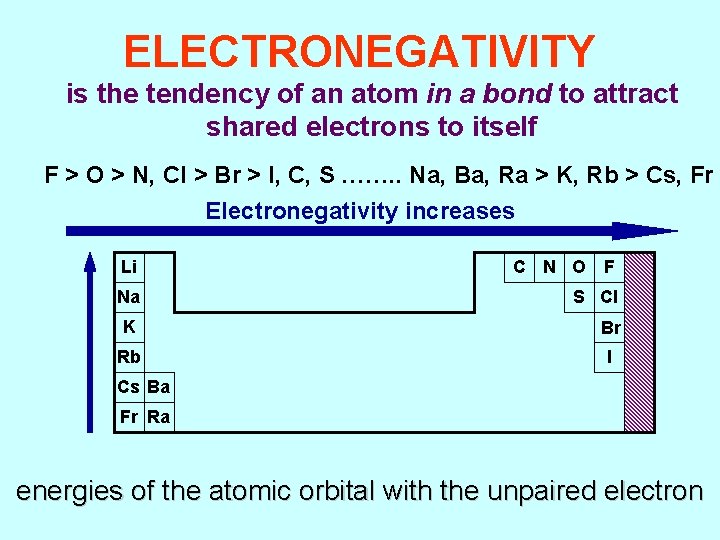
ELECTRONEGATIVITY is the tendency of an atom in a bond to attract shared electrons to itself F > O > N, Cl > Br > I, C, S ……. . Na, Ba, Ra > K, Rb > Cs, Fr Electronegativity increases Li C N O F Na S Cl K Br Rb I Cs Ba Fr Ra energies of the atomic orbital with the unpaired electron
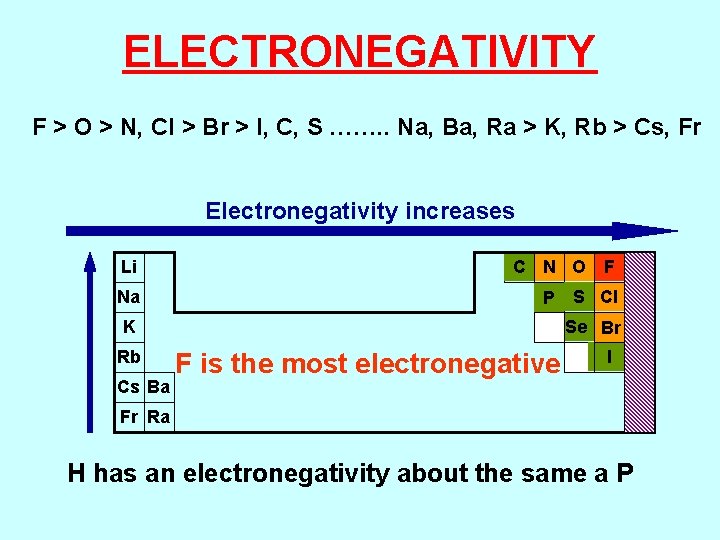
ELECTRONEGATIVITY F > O > N, Cl > Br > I, C, S ……. . Na, Ba, Ra > K, Rb > Cs, Fr Electronegativity increases Li Na C N O F P Se Br K Rb Cs Ba S Cl F is the most electronegative I Fr Ra H has an electronegativity about the same a P

IONIC VERSUS COVALENT BONDS bonds are neither completely ionic nor covalent (only in homonuclear molecules)
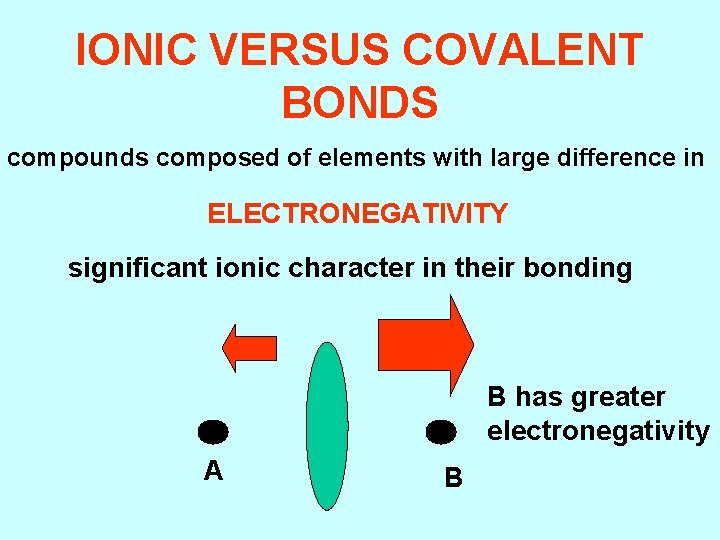
IONIC VERSUS COVALENT BONDS compounds composed of elements with large difference in ELECTRONEGATIVITY significant ionic character in their bonding B has greater electronegativity A B
IONIC VERSUS COVALENT BONDS B has a greater share A B
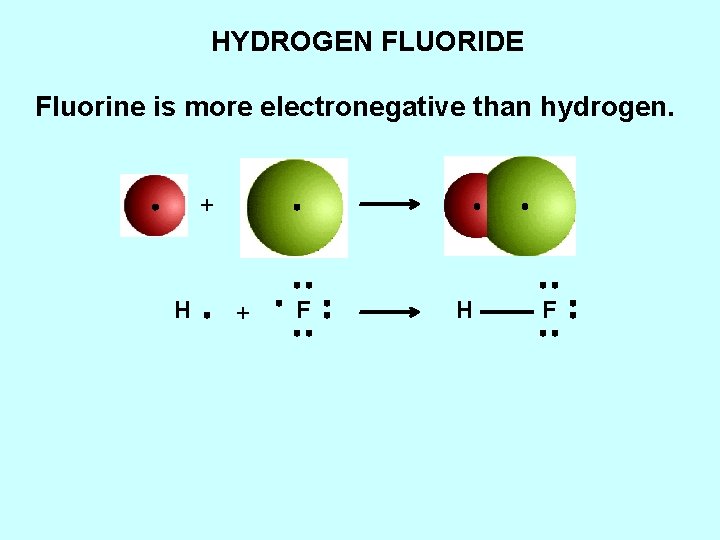
HYDROGEN FLUORIDE Fluorine is more electronegative than hydrogen. + H + F H F
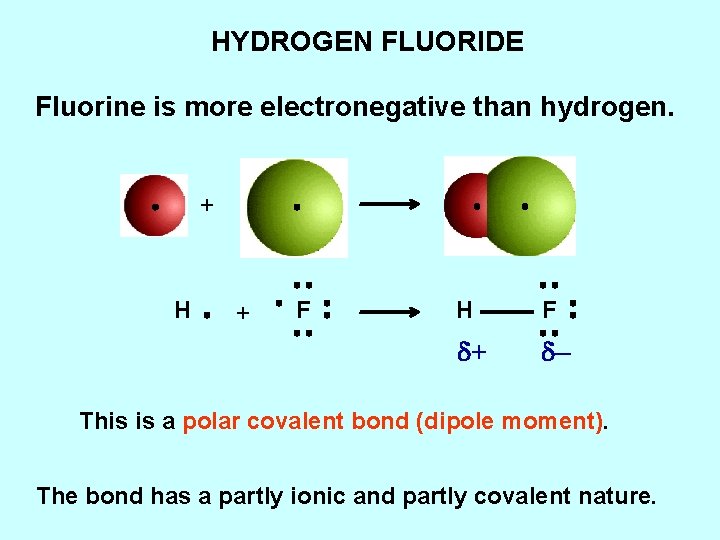
HYDROGEN FLUORIDE Fluorine is more electronegative than hydrogen. + H + F H F d+ d– This is a polar covalent bond (dipole moment). The bond has a partly ionic and partly covalent nature.
Microwave Spectroscopy molecules need a dipole moment
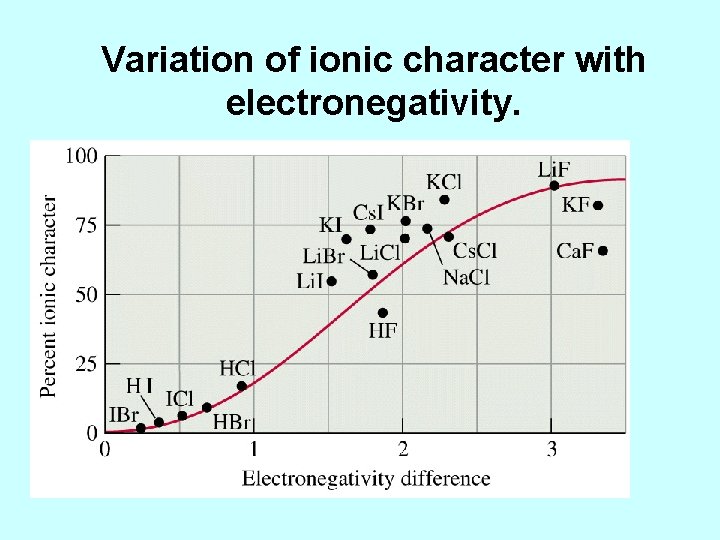
Variation of ionic character with electronegativity.
LEWIS SYMBOLS IONIC COMPOUDS COVALENT COMPOUNDS ELECTRONEGATIVITY
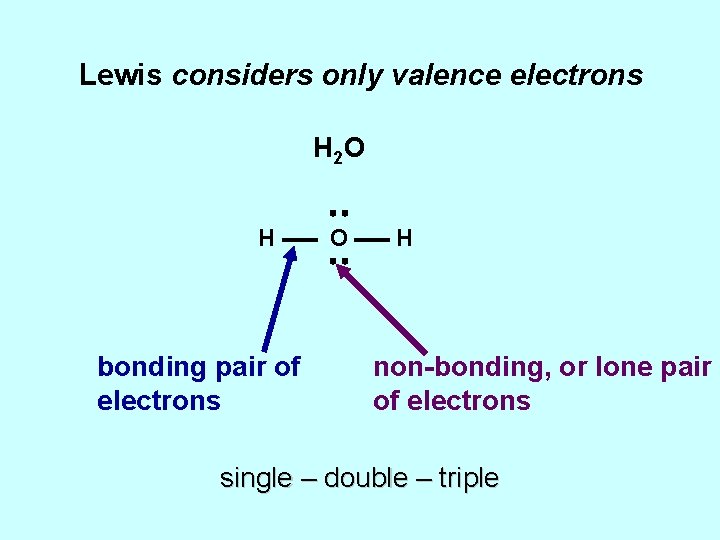
Lewis considers only valence electrons H 2 O H bonding pair of electrons O H non-bonding, or lone pair of electrons single – double – triple
LEWIS STRUCTURES 1. concept of resonances 2. exceptions to the octet rule
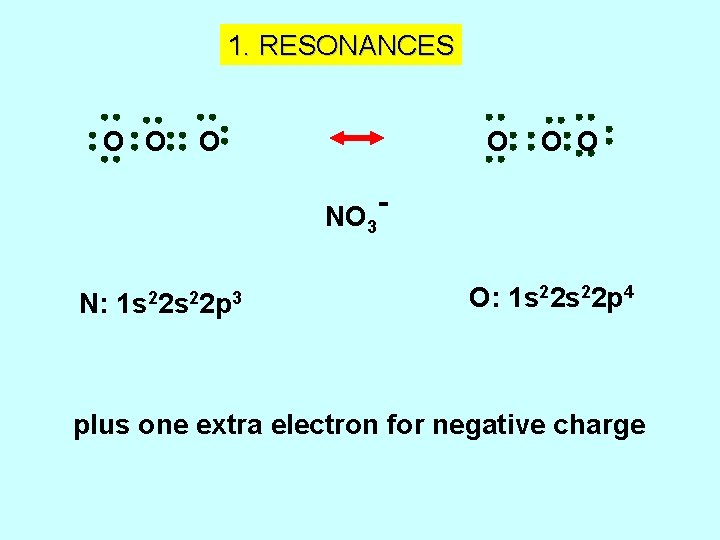
1. RESONANCES O O NO 3 N: 1 s 22 p 3 O O O: 1 s 22 p 4 plus one extra electron for negative charge
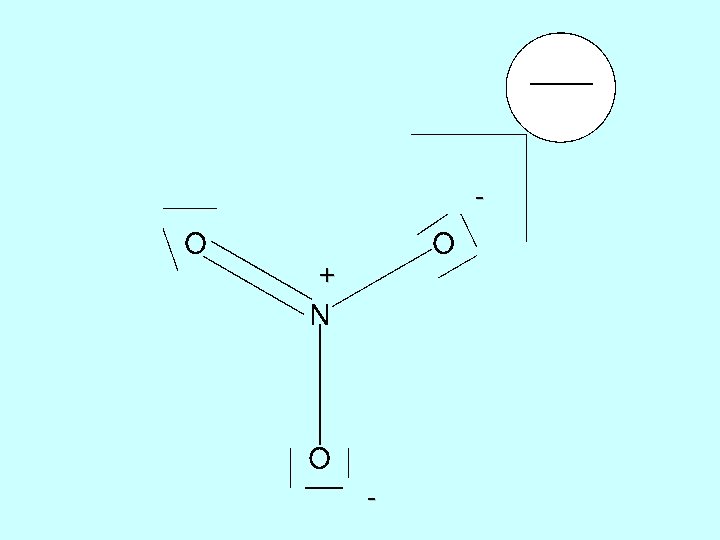
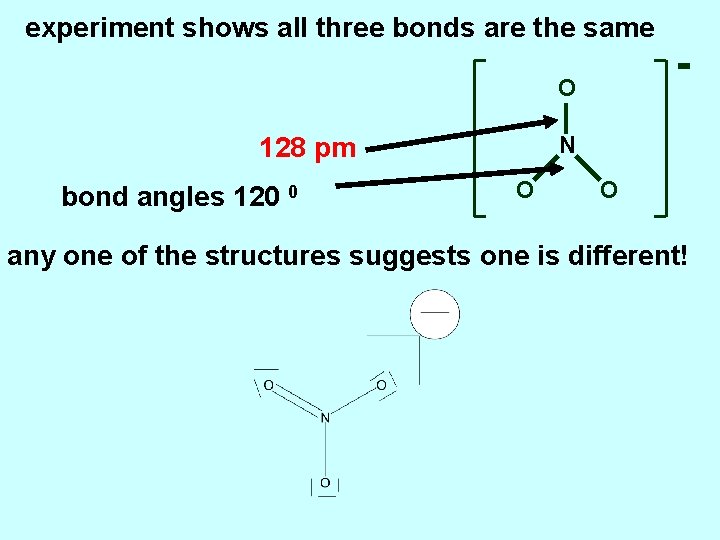
experiment shows all three bonds are the same O N 128 pm bond angles 120 0 O O any one of the structures suggests one is different!
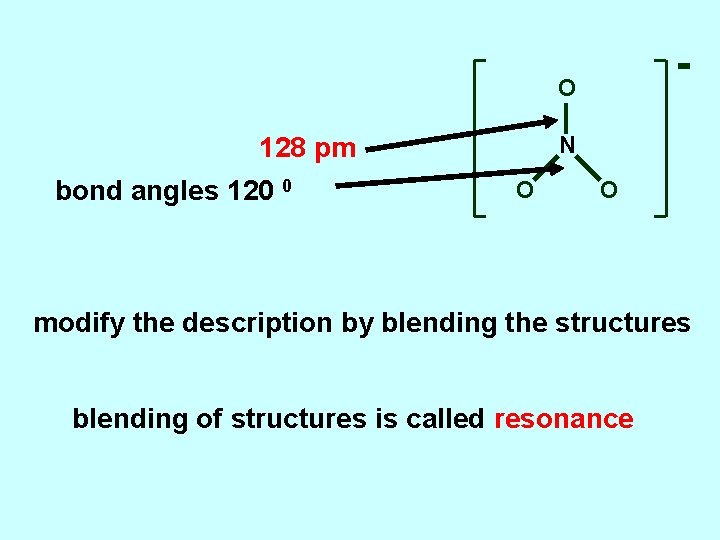
O N 128 pm bond angles 120 0 O O modify the description by blending the structures blending of structures is called resonance
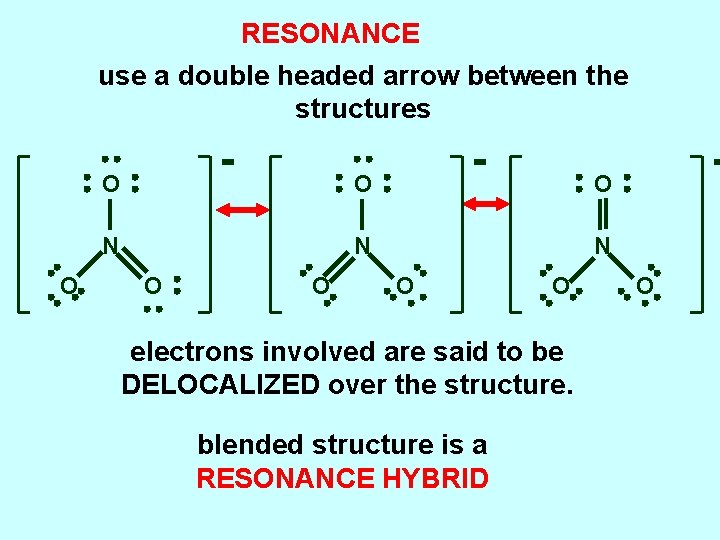
RESONANCE use a double headed arrow between the structures O O N N N O O electrons involved are said to be DELOCALIZED over the structure. blended structure is a RESONANCE HYBRID O
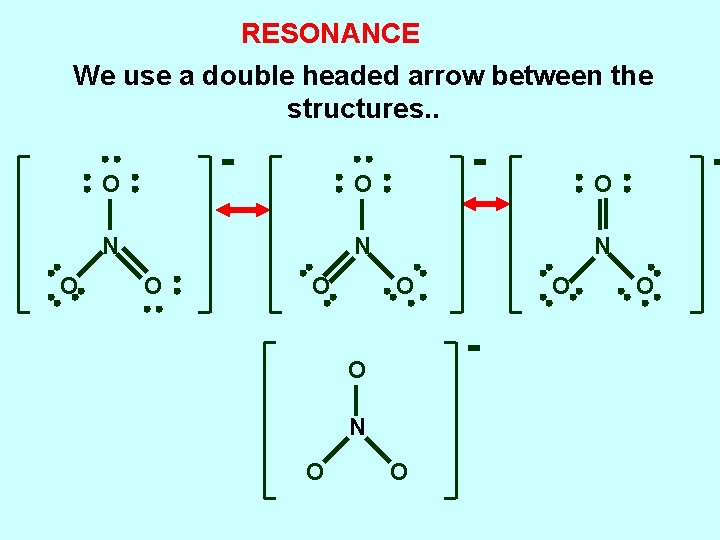
RESONANCE We use a double headed arrow between the structures. . O O N N N O O O O
2. Exceptions to the octet rule 1. more than 8 electrons around central atom 2. less than an octet around central atom 3. molecules with unpaired electrons
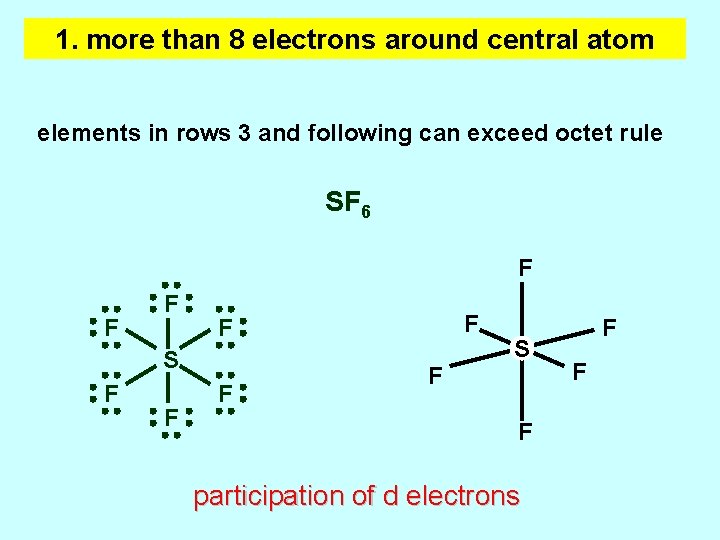
1. more than 8 electrons around central atom elements in rows 3 and following can exceed octet rule SF 6 F F F S F F participation of d electrons F F
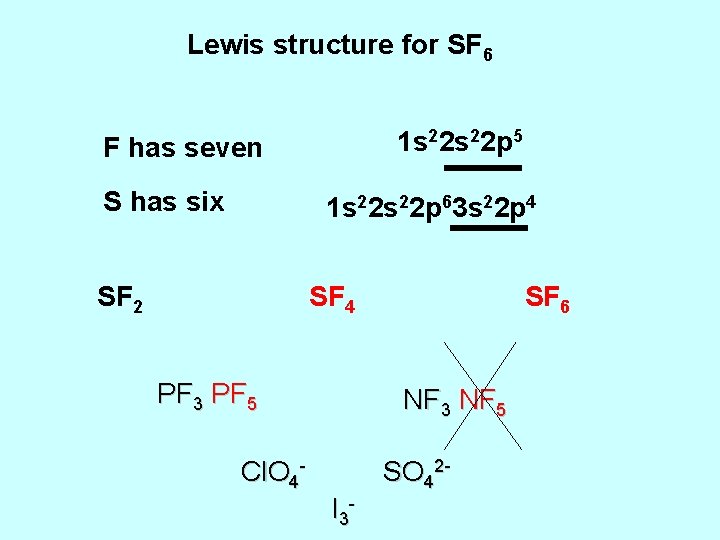
Lewis structure for SF 6 1 s 22 p 5 F has seven S has six 1 s 22 p 63 s 22 p 4 SF 2 SF 4 PF 3 PF 5 SF 6 NF 3 NF 5 Cl. O 4 - SO 42 I 3 -
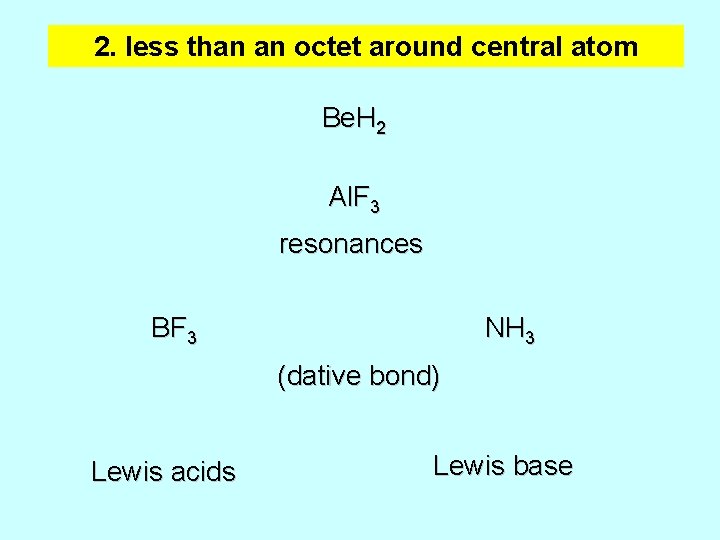
2. less than an octet around central atom Be. H 2 Al. F 3 resonances BF 3 NH 3 (dative bond) Lewis acids Lewis base

3. molecules with unpaired electrons FREE RADICALS NO but not NO
- Slides: 45