CHEMISTRY 161 Chapter 6 www chem hawaii eduBil

CHEMISTRY 161 Chapter 6 www. chem. hawaii. edu/Bil 301/welcome. html
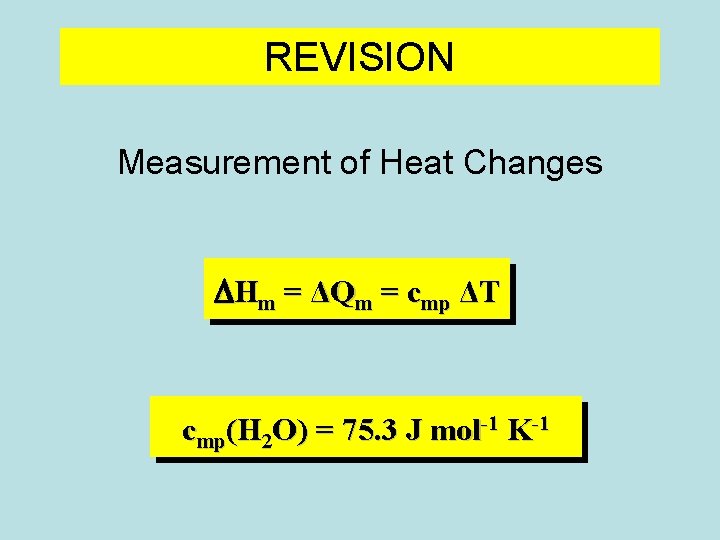
REVISION Measurement of Heat Changes DHm = ΔQm = cmp ΔT cmp(H 2 O) = 75. 3 J mol-1 K-1
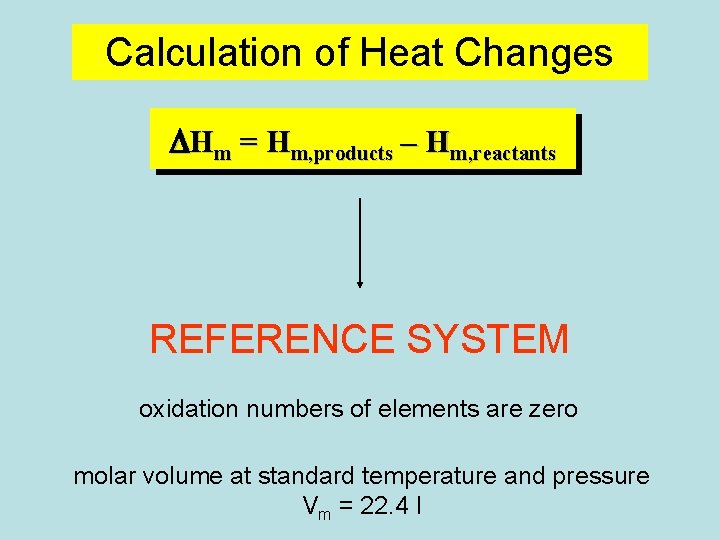
Calculation of Heat Changes DHm = Hm, products – Hm, reactants REFERENCE SYSTEM oxidation numbers of elements are zero molar volume at standard temperature and pressure Vm = 22. 4 l
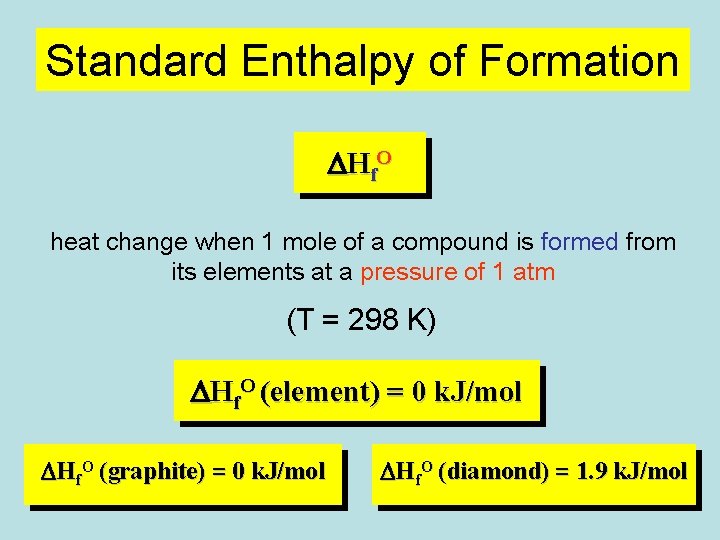
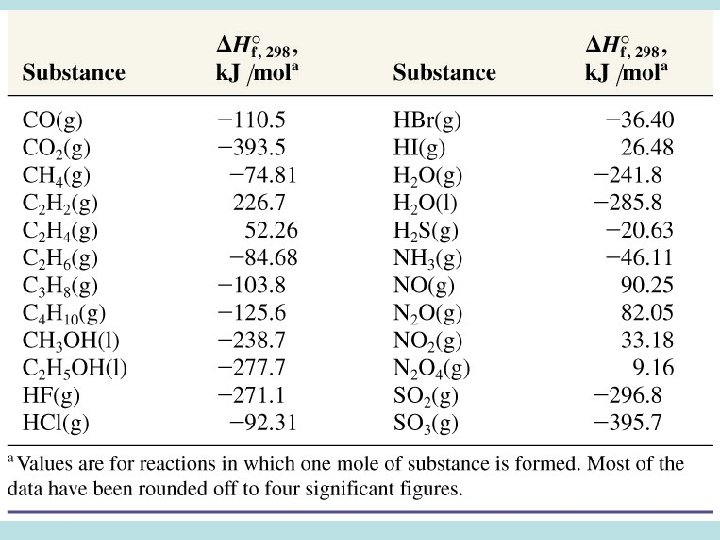
Standard Enthalpy of Formation D H f. O heat change when 1 mole of a compound is formed from its elements at a pressure of 1 atm (T = 298 K) DHf. O (element) = 0 k. J/mol DHf. O (graphite) = 0 k. J/mol DHf. O (diamond) = 1. 9 k. J/mol
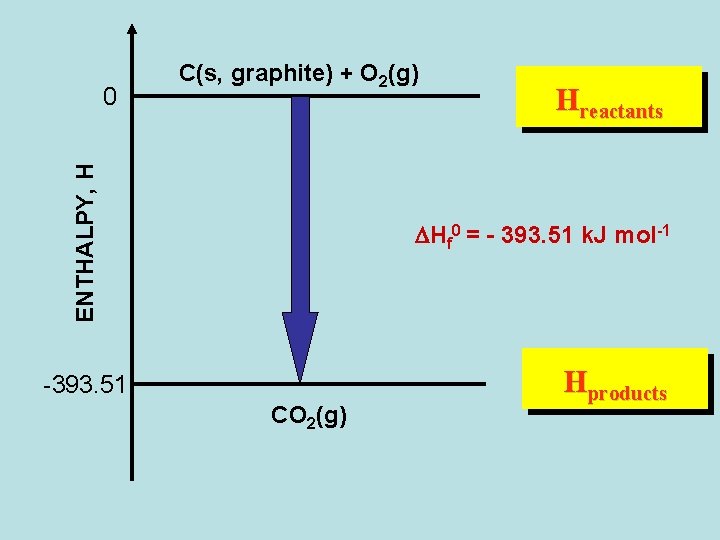
ENTHALPY, H 0 C(s, graphite) + O 2(g) Hreactants DHf 0 = - 393. 51 k. J mol-1 -393. 51 CO 2(g) Hproducts
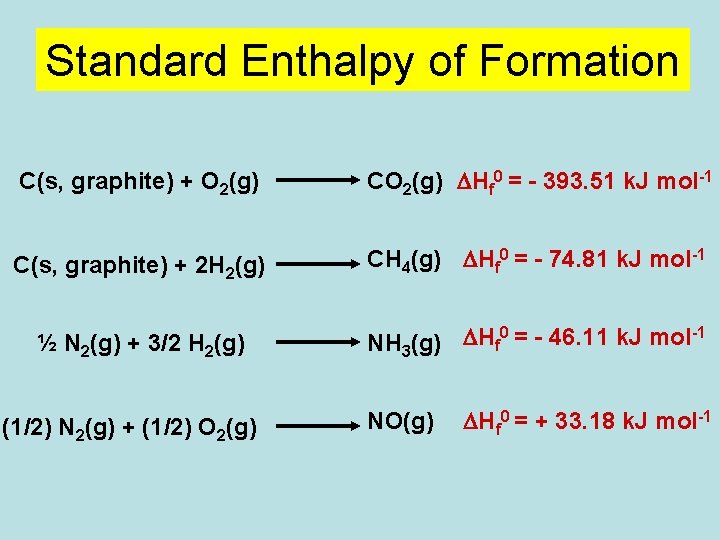
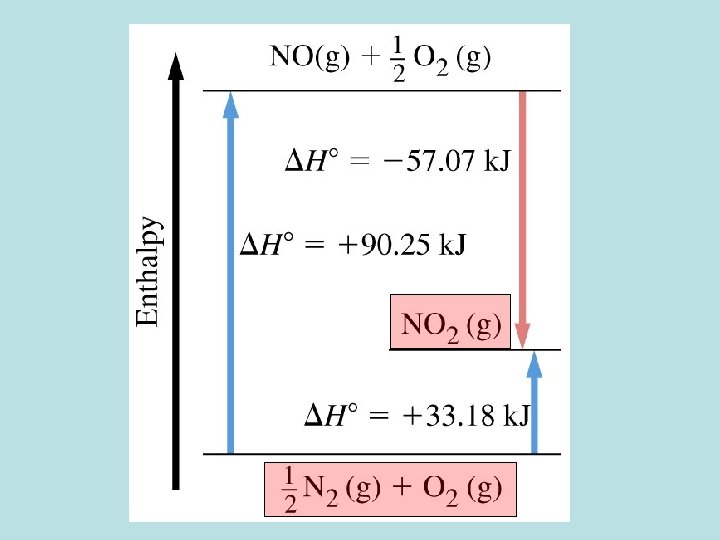
Standard Enthalpy of Formation C(s, graphite) + O 2(g) CO 2(g) DHf 0 = - 393. 51 k. J mol-1 C(s, graphite) + 2 H 2(g) CH 4(g) DHf 0 = - 74. 81 k. J mol-1 ½ N 2(g) + 3/2 H 2(g) 0 -1 NH 3(g) DHf = - 46. 11 k. J mol (1/2) N 2(g) + (1/2) O 2(g) NO(g) DHf 0 = + 33. 18 k. J mol-1

Standard Enthalpy of Reaction a. A+b. B→c. C+d. D ENTHALPY, H a. A+b. B a × DHf. O (A) + b × ΔHf. O(B) Hreactants DHOrxn = ΣΔHf 0(prod) – ΣΔHf 0(react) Hproducts c. C+d. D c × DHf. O(C) + d × ΔHf. O(D)
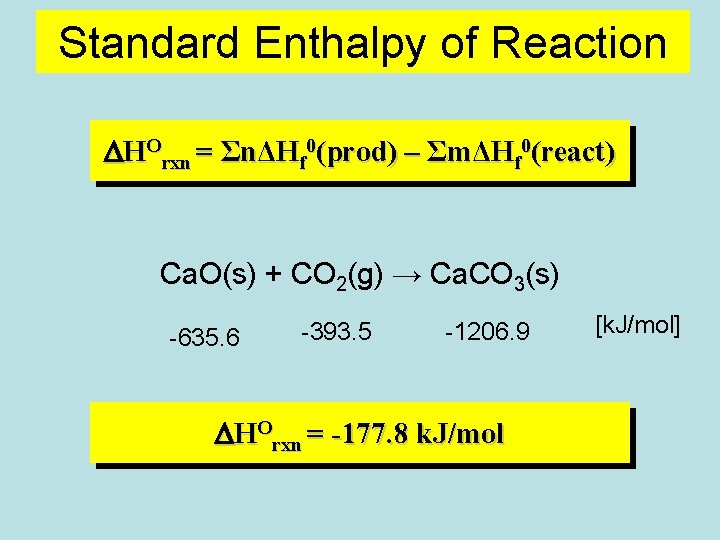
Standard Enthalpy of Reaction DHOrxn = ΣnΔHf 0(prod) – ΣmΔHf 0(react) Ca. O(s) + CO 2(g) → Ca. CO 3(s) -635. 6 -393. 5 -1206. 9 DHOrxn = -177. 8 k. J/mol [k. J/mol]
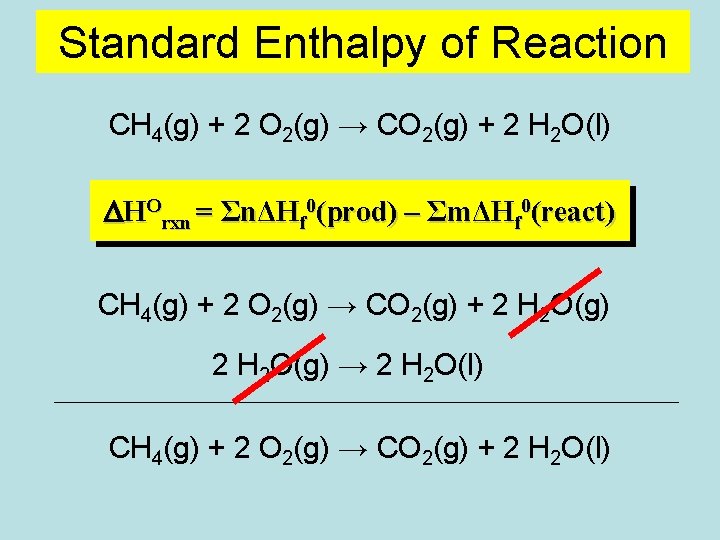
Standard Enthalpy of Reaction CH 4(g) + 2 O 2(g) → CO 2(g) + 2 H 2 O(l) DHOrxn = ΣnΔHf 0(prod) – ΣmΔHf 0(react) CH 4(g) + 2 O 2(g) → CO 2(g) + 2 H 2 O(g) → 2 H 2 O(l) CH 4(g) + 2 O 2(g) → CO 2(g) + 2 H 2 O(l)
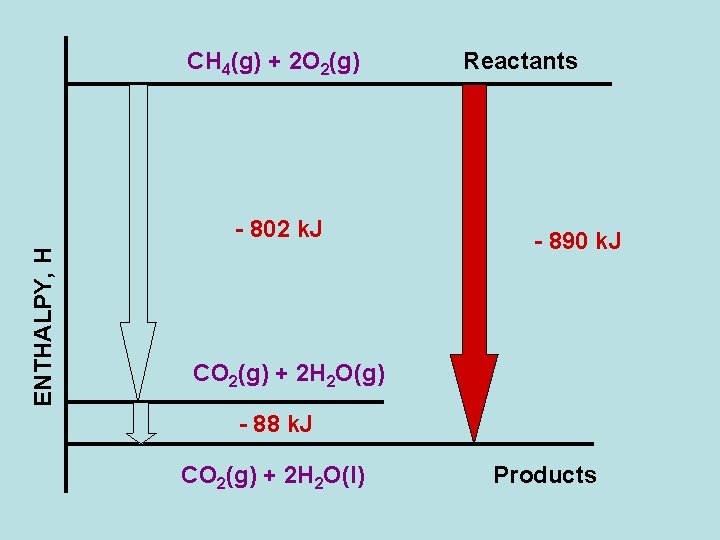
CH 4(g) + 2 O 2(g) ENTHALPY, H - 802 k. J Reactants - 890 k. J CO 2(g) + 2 H 2 O(g) - 88 k. J CO 2(g) + 2 H 2 O(l) Products

Hess’s Law The overall reaction enthalpy is the sum of the reaction enthalpies of the steps in which the reaction can be divided
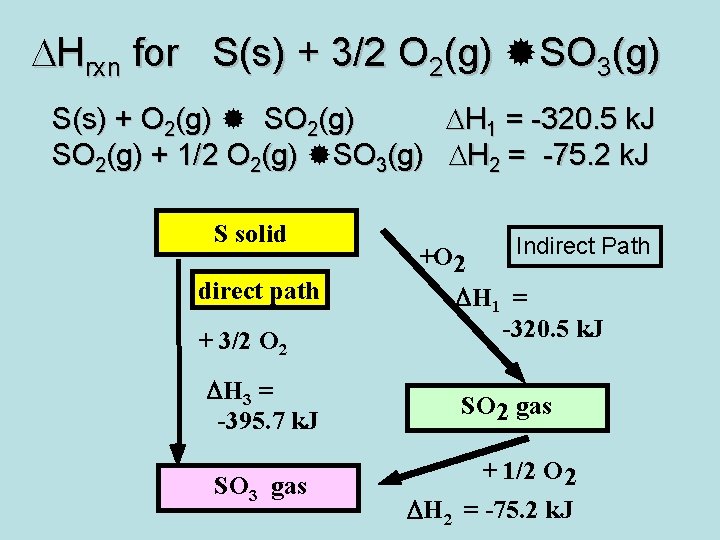
DHrxn for S(s) + 3/2 O 2(g) SO 3(g) S(s) + O 2(g) SO 2(g) DH 1 = -320. 5 k. J SO 2(g) + 1/2 O 2(g) SO 3(g) DH 2 = -75. 2 k. J S solid direct path + 3/2 O 2 DH 3 = -395. 7 k. J SO 3 gas +O 2 Indirect Path DH 1 = -320. 5 k. J SO 2 gas + 1/2 O 2 DH 2 = -75. 2 k. J
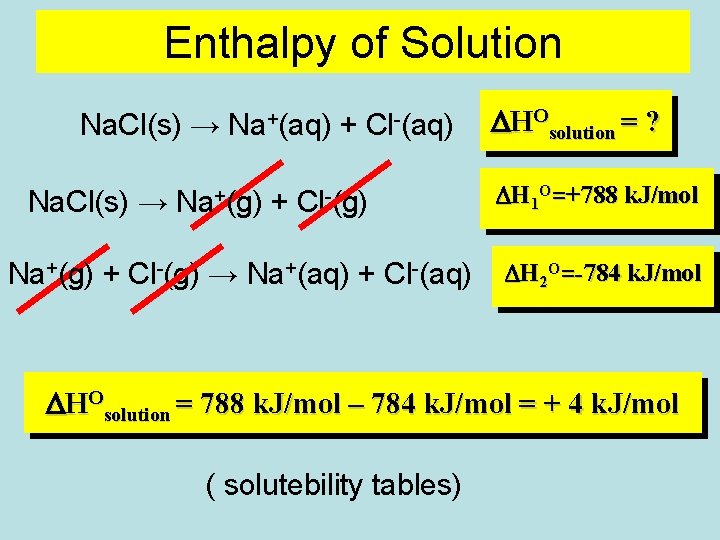
Enthalpy of Solution Na. Cl(s) → Na+(aq) + Cl-(aq) Na. Cl(s) → Na+(g) + Cl-(g) → Na+(aq) + Cl-(aq) DHOsolution = ? DH 1 O=+788 k. J/mol DH 2 O=-784 k. J/mol DHOsolution = 788 k. J/mol – 784 k. J/mol = + 4 k. J/mol ( solutebility tables)
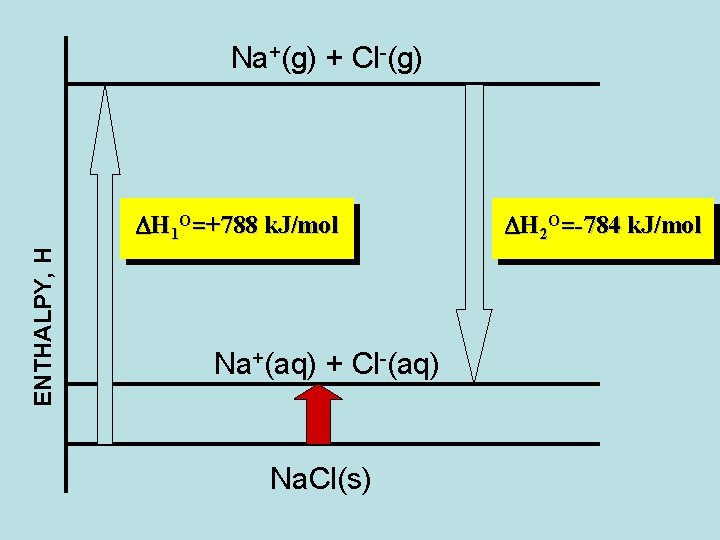
Na+(g) + Cl-(g) ENTHALPY, H DH 1 O=+788 k. J/mol Na+(aq) + Cl-(aq) Na. Cl(s) DH 2 O=-784 k. J/mol
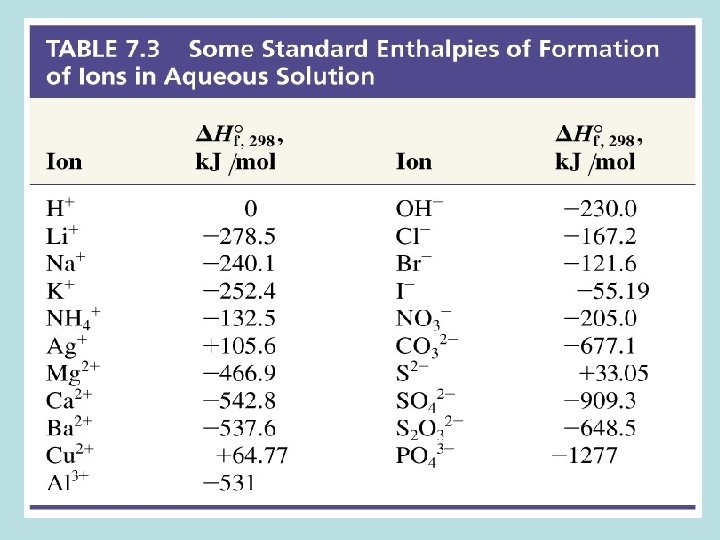
SUMMARY Standard Enthalpy of Formation D H f. O DHf. O (element) = 0 k. J/mol Standard Enthalpy of Reaction Hess’s Law

Homework Chapter 6, p. 217 -222 problems
- Slides: 19