CHEMISTRY 161 Chapter 4 www chem hawaii eduBil

CHEMISTRY 161 Chapter 4 www. chem. hawaii. edu/Bil 301/welcome. html ‘ 4’

REVISION 1. redox reactions 2. 2. oxidation versus reduction 3. 3. oxidation numbers versus charges 4. 4. calculation of oxidation numbers
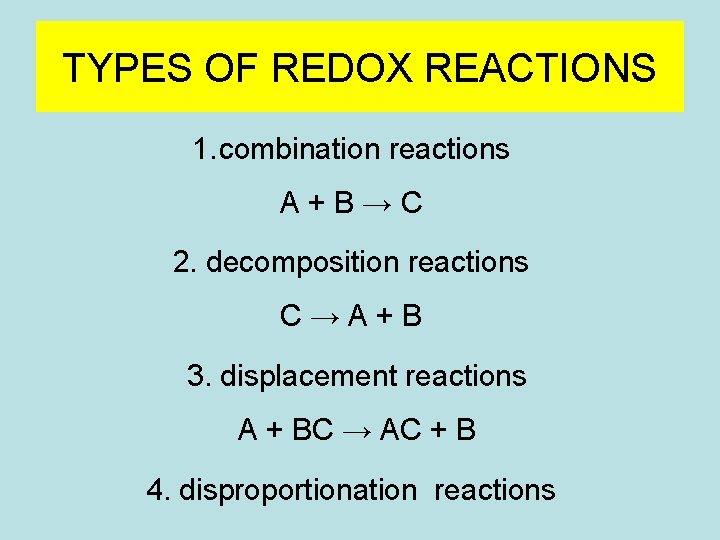
TYPES OF REDOX REACTIONS 1. combination reactions A+B→C 2. decomposition reactions C→A+B 3. displacement reactions A + BC → AC + B 4. disproportionation reactions
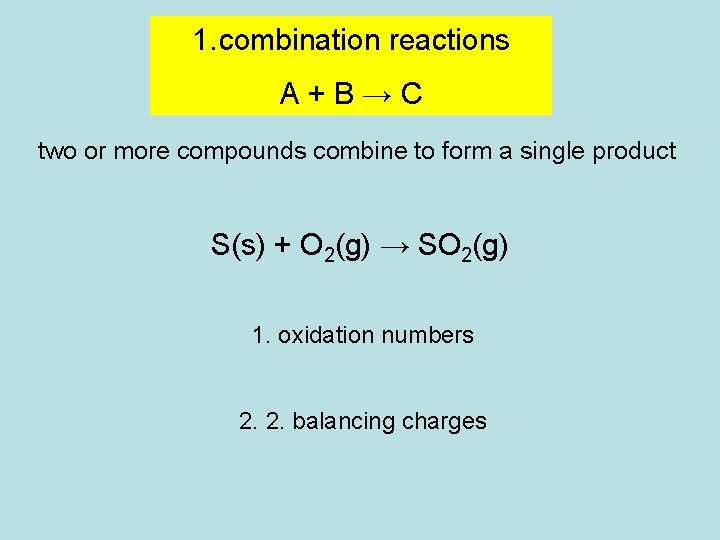
1. combination reactions A+B→C two or more compounds combine to form a single product S(s) + O 2(g) → SO 2(g) 1. oxidation numbers 2. 2. balancing charges

MENUE 1. oxidation states of group I – III metals 2. oxidation state of hydrogen (+1, -1) 3. oxidation states of oxygen (-2, -1/2, +1) 4. oxidation state of halogens 5. remaining atoms
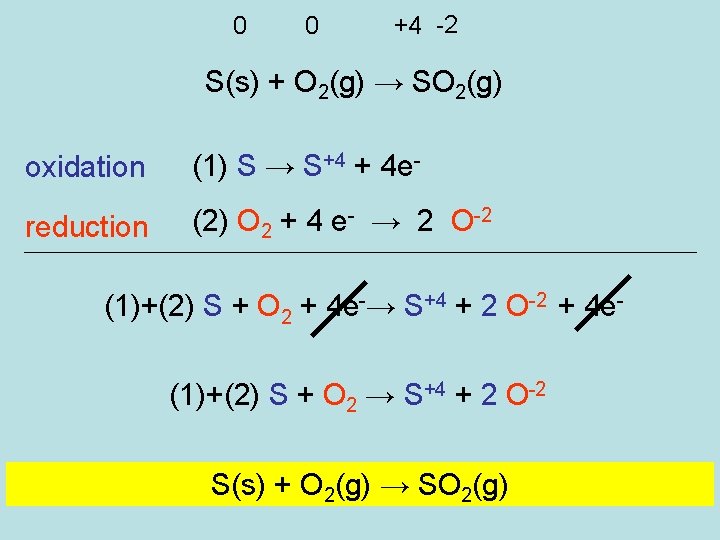
0 0 +4 -2 S(s) + O 2(g) → SO 2(g) oxidation (1) S → S+4 + 4 e- reduction (2) O 2 + 4 e- → 2 O-2 (1)+(2) S + O 2 + 4 e-→ S+4 + 2 O-2 + 4 e(1)+(2) S + O 2 → S+4 + 2 O-2 S(s) + O 2(g) → SO 2(g)
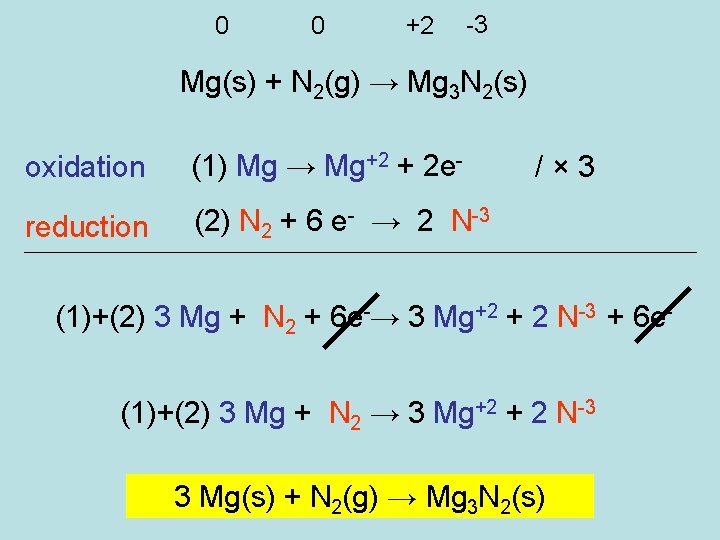
0 0 +2 -3 Mg(s) + N 2(g) → Mg 3 N 2(s) oxidation (1) Mg → Mg+2 + 2 e- reduction (2) N 2 + 6 e- → 2 N-3 /× 3 (1)+(2) 3 Mg + N 2 + 6 e-→ 3 Mg+2 + 2 N-3 + 6 e(1)+(2) 3 Mg + N 2 → 3 Mg+2 + 2 N-3 3 Mg(s) + N 2(g) → Mg 3 N 2(s)
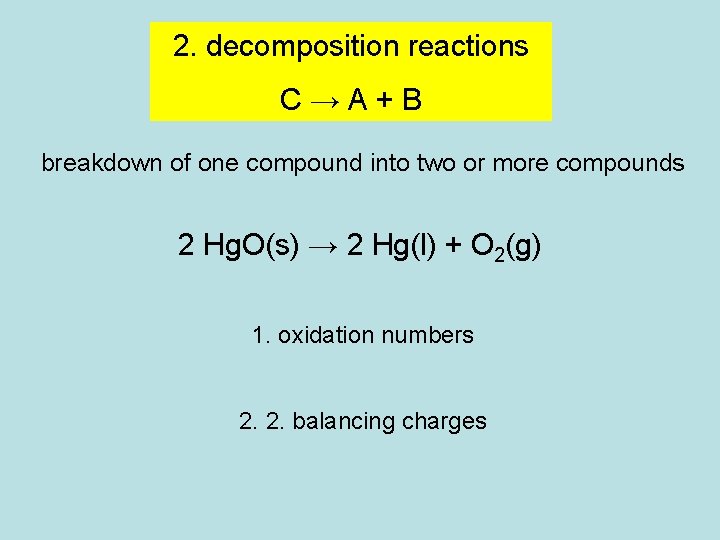
2. decomposition reactions C→A+B breakdown of one compound into two or more compounds 2 Hg. O(s) → 2 Hg(l) + O 2(g) 1. oxidation numbers 2. 2. balancing charges
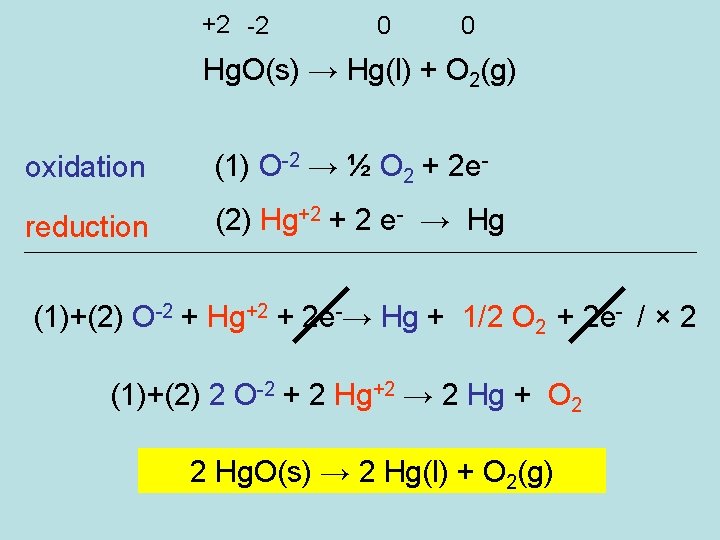
+2 -2 0 0 Hg. O(s) → Hg(l) + O 2(g) oxidation (1) O-2 → ½ O 2 + 2 e- reduction (2) Hg+2 + 2 e- → Hg (1)+(2) O-2 + Hg+2 + 2 e-→ Hg + 1/2 O 2 + 2 e- / × 2 (1)+(2) 2 O-2 + 2 Hg+2 → 2 Hg + O 2 2 Hg. O(s) → 2 Hg(l) + O 2(g)
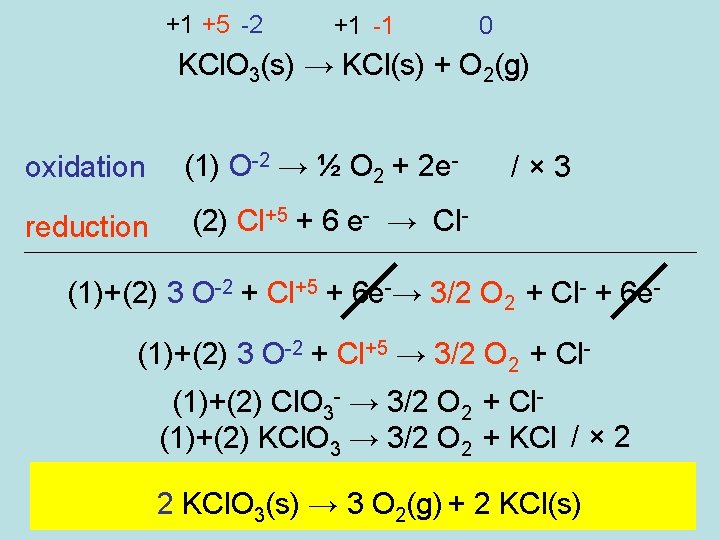
+1 +5 -2 +1 -1 0 KCl. O 3(s) → KCl(s) + O 2(g) oxidation (1) O-2 → ½ O 2 + 2 e- reduction (2) Cl+5 + 6 e- → Cl- /× 3 (1)+(2) 3 O-2 + Cl+5 + 6 e-→ 3/2 O 2 + Cl- + 6 e(1)+(2) 3 O-2 + Cl+5 → 3/2 O 2 + Cl(1)+(2) Cl. O 3 - → 3/2 O 2 + Cl(1)+(2) KCl. O 3 → 3/2 O 2 + KCl / × 2 2 KCl. O 3(s) → 3 O 2(g) + 2 KCl(s)
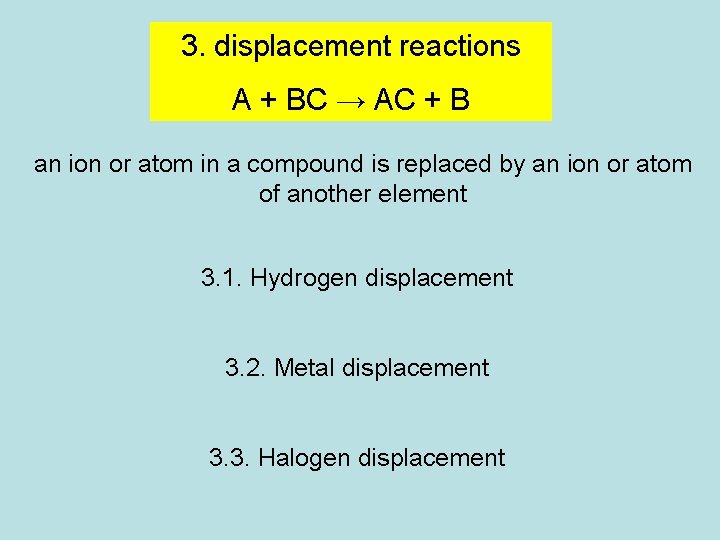
3. displacement reactions A + BC → AC + B an ion or atom in a compound is replaced by an ion or atom of another element 3. 1. Hydrogen displacement 3. 2. Metal displacement 3. 3. Halogen displacement
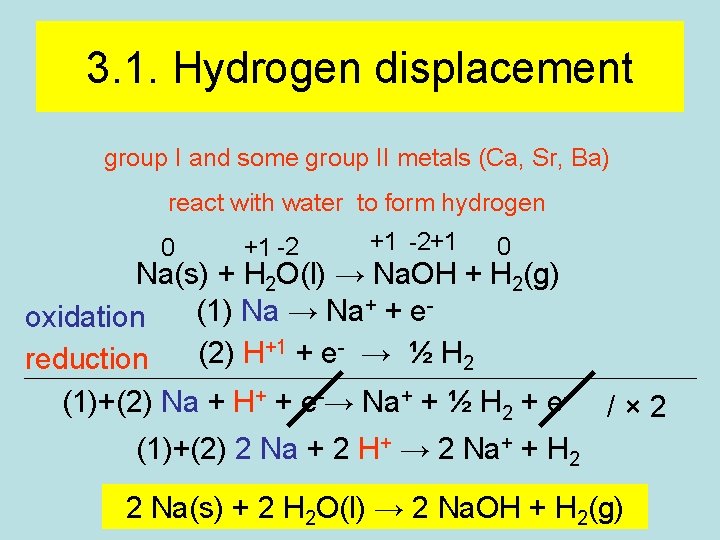
3. 1. Hydrogen displacement group I and some group II metals (Ca, Sr, Ba) react with water to form hydrogen 0 +1 -2+1 0 Na(s) + H 2 O(l) → Na. OH + H 2(g) + + e(1) Na → Na oxidation +1 + e- → ½ H (2) H reduction 2 (1)+(2) Na + H+ + e-→ Na+ + ½ H 2 + e- /× 2 (1)+(2) 2 Na + 2 H+ → 2 Na+ + H 2 2 Na(s) + 2 H 2 O(l) → 2 Na. OH + H 2(g)
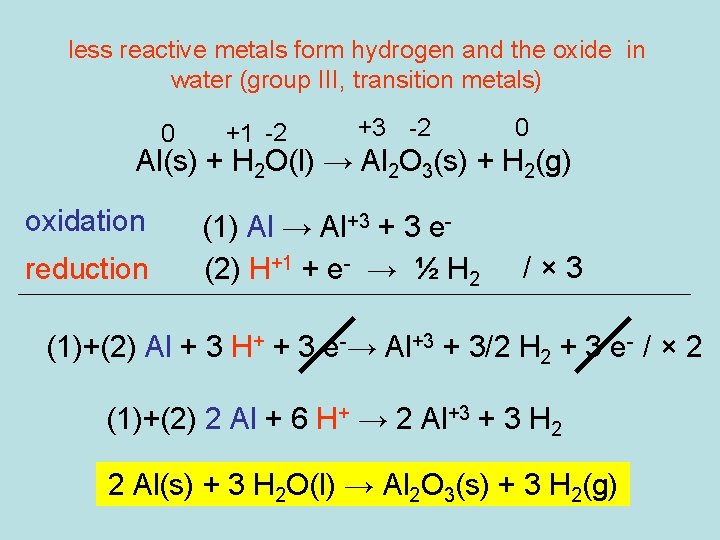
less reactive metals form hydrogen and the oxide in water (group III, transition metals) 0 +1 -2 +3 -2 0 Al(s) + H 2 O(l) → Al 2 O 3(s) + H 2(g) oxidation reduction (1) Al → Al+3 + 3 e(2) H+1 + e- → ½ H 2 /× 3 (1)+(2) Al + 3 H+ + 3 e-→ Al+3 + 3/2 H 2 + 3 e- / × 2 (1)+(2) 2 Al + 6 H+ → 2 Al+3 + 3 H 2 2 Al(s) + 3 H 2 O(l) → Al 2 O 3(s) + 3 H 2(g)
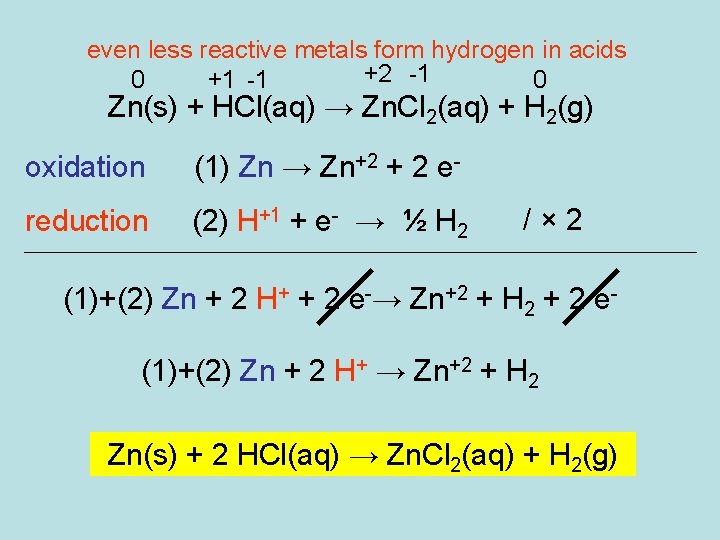
even less reactive metals form hydrogen in acids +2 -1 0 +1 -1 0 Zn(s) + HCl(aq) → Zn. Cl 2(aq) + H 2(g) oxidation (1) Zn → Zn+2 + 2 e- reduction (2) H+1 + e- → ½ H 2 /× 2 (1)+(2) Zn + 2 H+ + 2 e-→ Zn+2 + H 2 + 2 e(1)+(2) Zn + 2 H+ → Zn+2 + H 2 Zn(s) + 2 HCl(aq) → Zn. Cl 2(aq) + H 2(g)
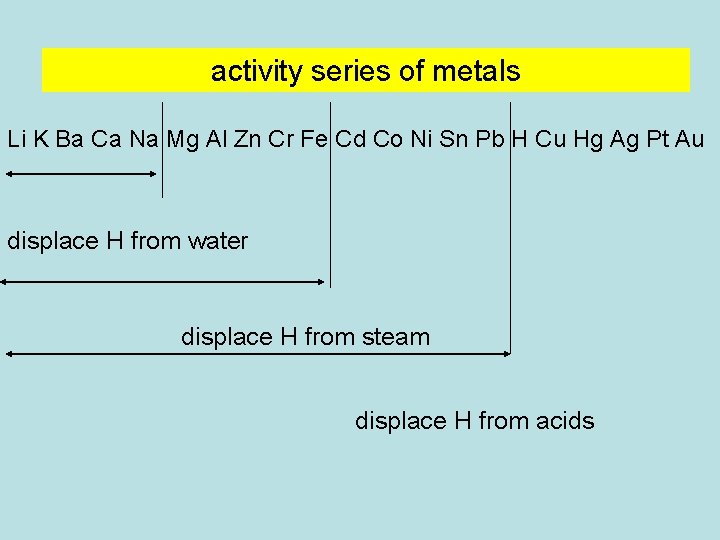
activity series of metals Li K Ba Ca Na Mg Al Zn Cr Fe Cd Co Ni Sn Pb H Cu Hg Ag Pt Au displace H from water displace H from steam displace H from acids

3. 2. Metal displacement V 2 O 5(s) + 5 Ca(s) → 2 V(s) + 5 Ca. O(s) Ti. Cl 4(g) + 2 Mg (l) → Ti(s) + 2 Mg. Cl 2(l)
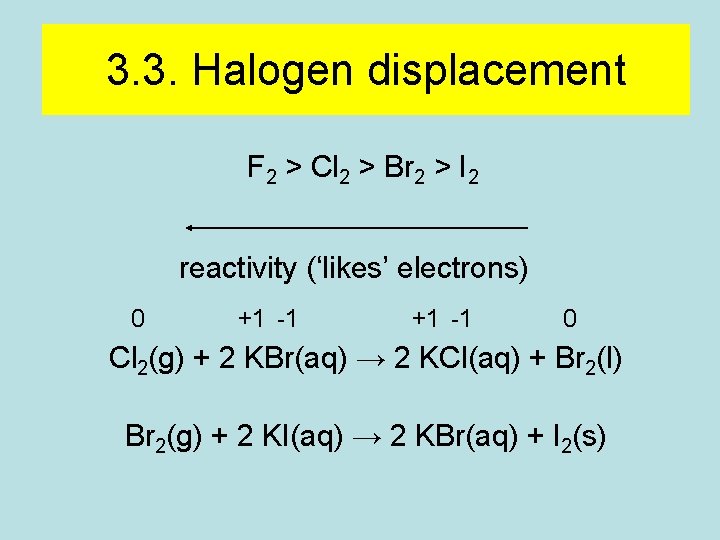
3. 3. Halogen displacement F 2 > Cl 2 > Br 2 > I 2 reactivity (‘likes’ electrons) 0 +1 -1 0 Cl 2(g) + 2 KBr(aq) → 2 KCl(aq) + Br 2(l) Br 2(g) + 2 KI(aq) → 2 KBr(aq) + I 2(s)
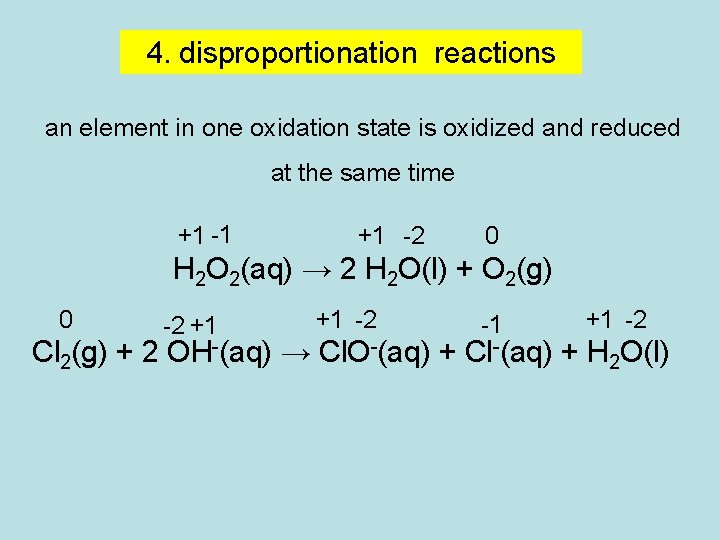
4. disproportionation reactions an element in one oxidation state is oxidized and reduced at the same time +1 -1 +1 -2 0 H 2 O 2(aq) → 2 H 2 O(l) + O 2(g) 0 -2 +1 +1 -2 -1 +1 -2 Cl 2(g) + 2 OH-(aq) → Cl. O-(aq) + Cl-(aq) + H 2 O(l)
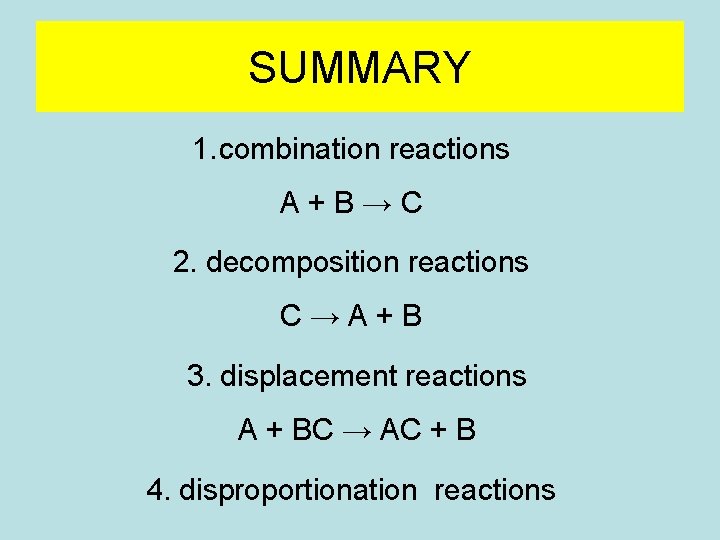
SUMMARY 1. combination reactions A+B→C 2. decomposition reactions C→A+B 3. displacement reactions A + BC → AC + B 4. disproportionation reactions

Homework Chapter 4, p. 121 -129 problems
- Slides: 20