Chemistry 142 Chapter 18 Free Energy and Thermodynamics


- Slides: 35

Chemistry 142 Chapter 18: Free Energy and Thermodynamics Outline I. First Law of Thermodynamics II. Entropy III. Second Law of Thermodynamics IV. Third Law of Thermodynamics V. Gibbs Free Energy

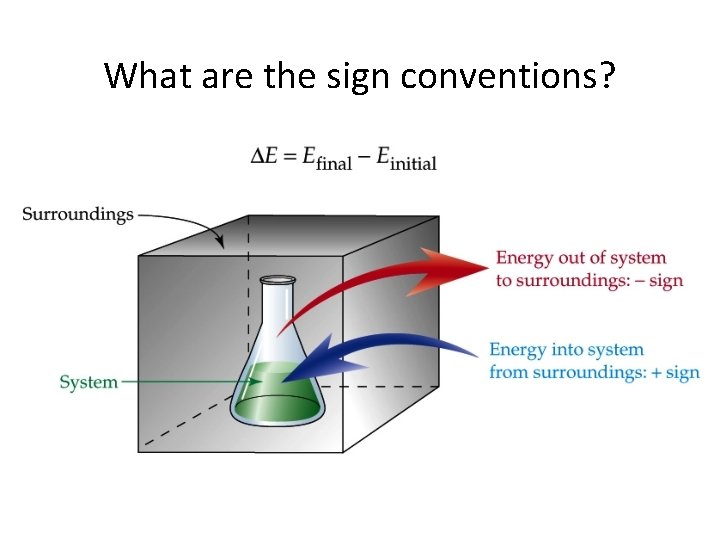
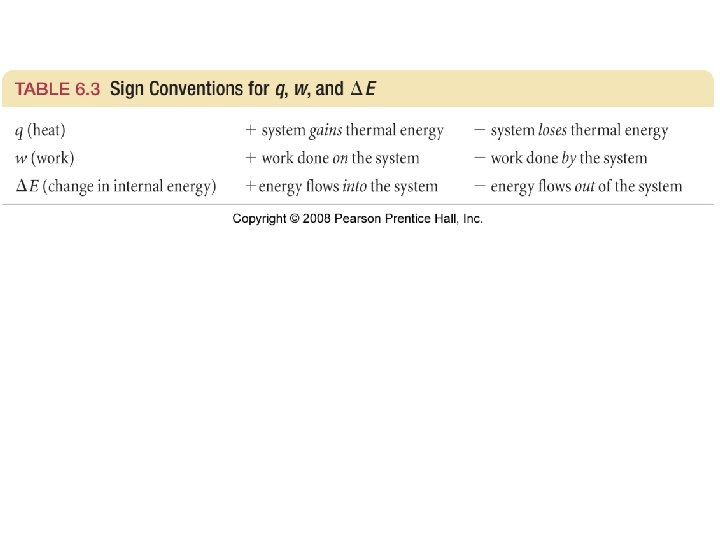
What are the sign conventions?

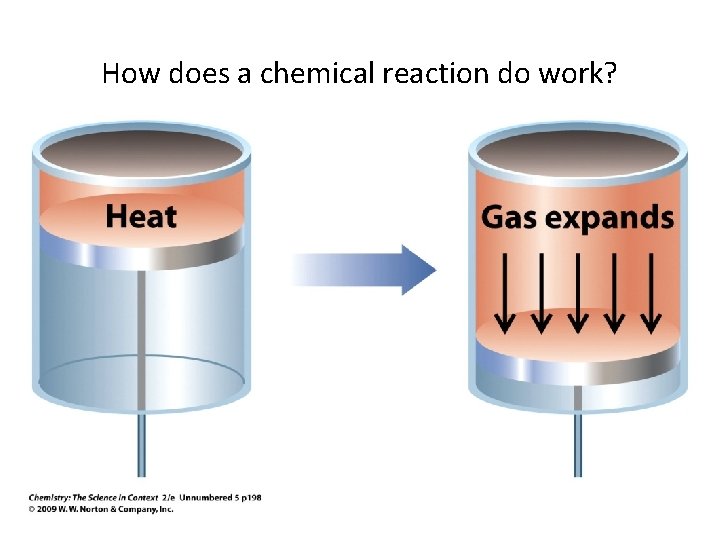
How does a chemical reaction do work?


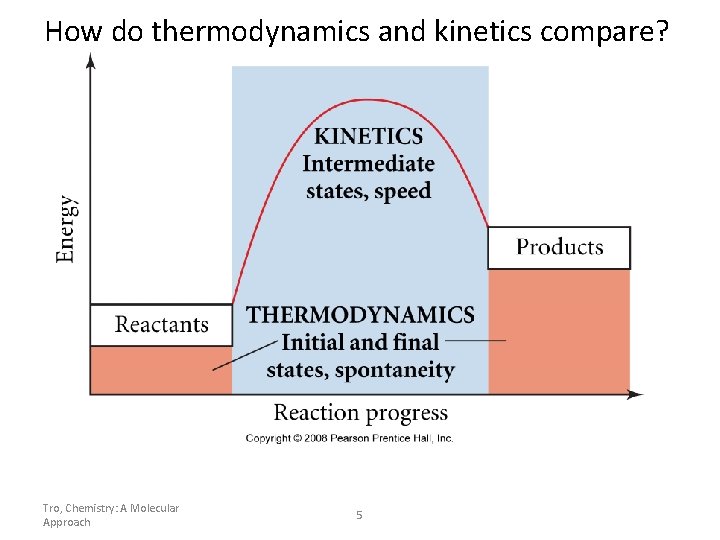
How do thermodynamics and kinetics compare? Tro, Chemistry: A Molecular Approach 5

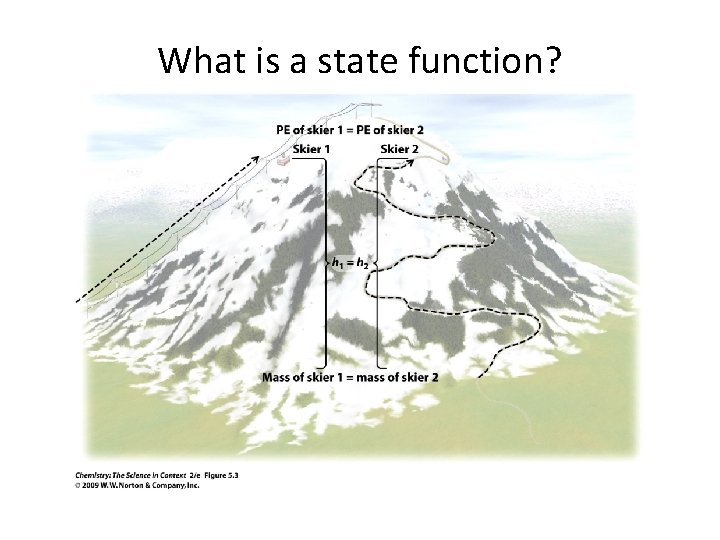
What is a state function?

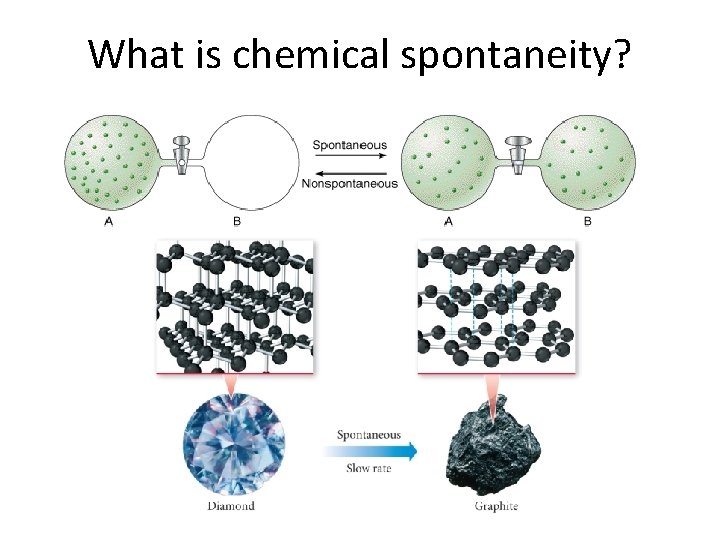
What is chemical spontaneity?

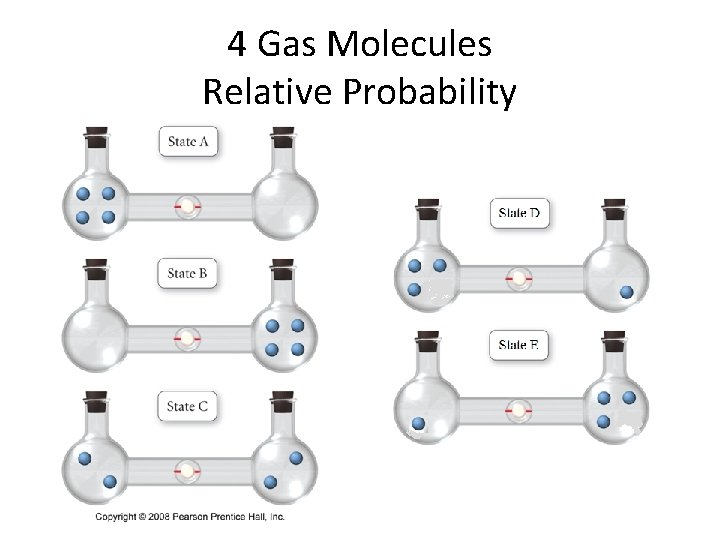
4 Gas Molecules Relative Probability

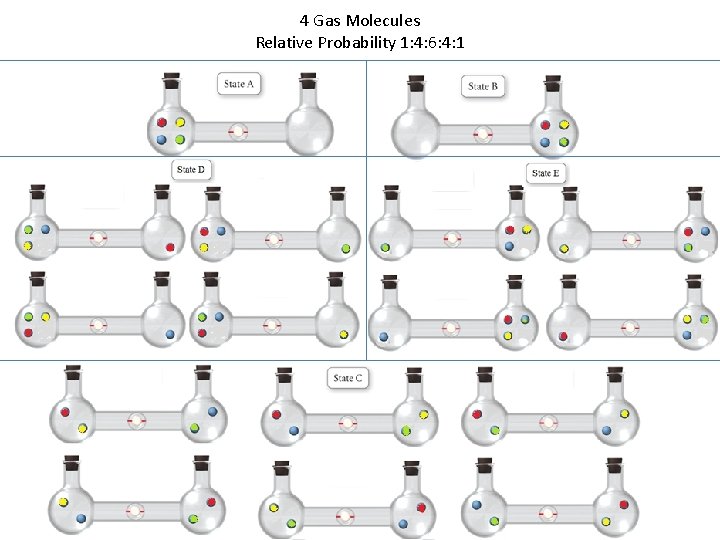
4 Gas Molecules Relative Probability 1: 4: 6: 4: 1

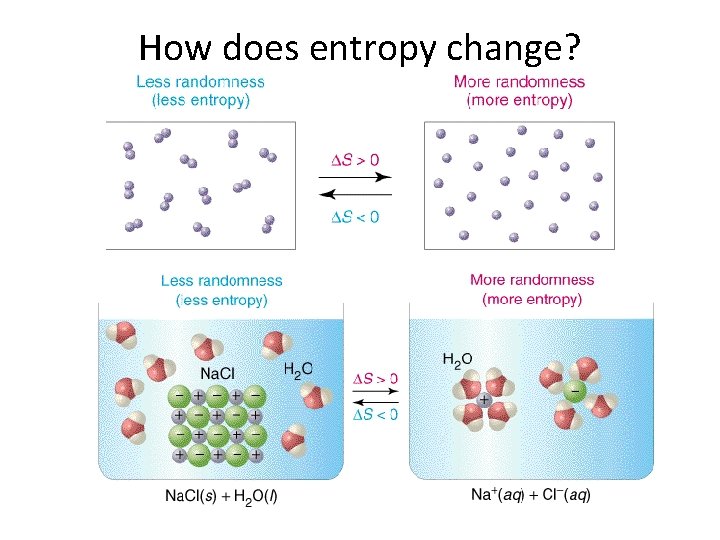
How does entropy change?

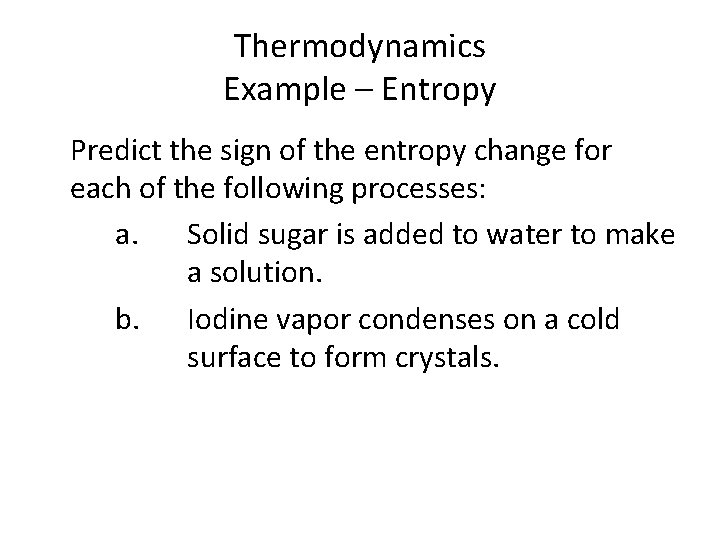
Thermodynamics Example – Entropy Predict the sign of the entropy change for each of the following processes: a. Solid sugar is added to water to make a solution. b. Iodine vapor condenses on a cold surface to form crystals.

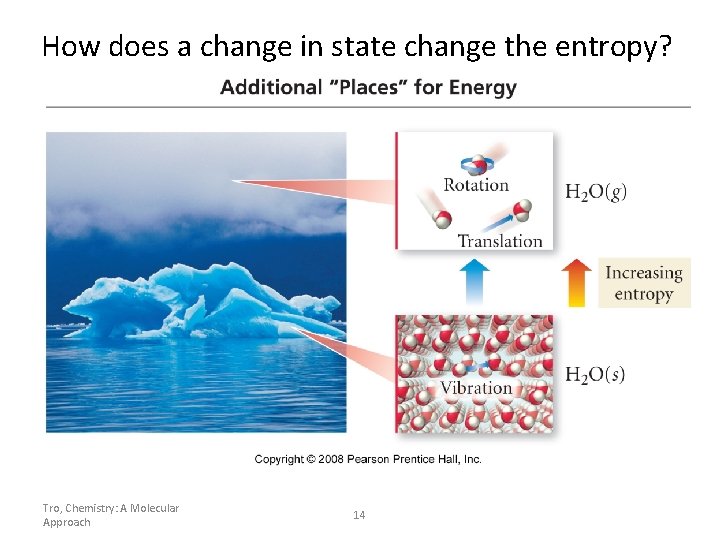
How does a change in state change the entropy? Tro, Chemistry: A Molecular Approach 14

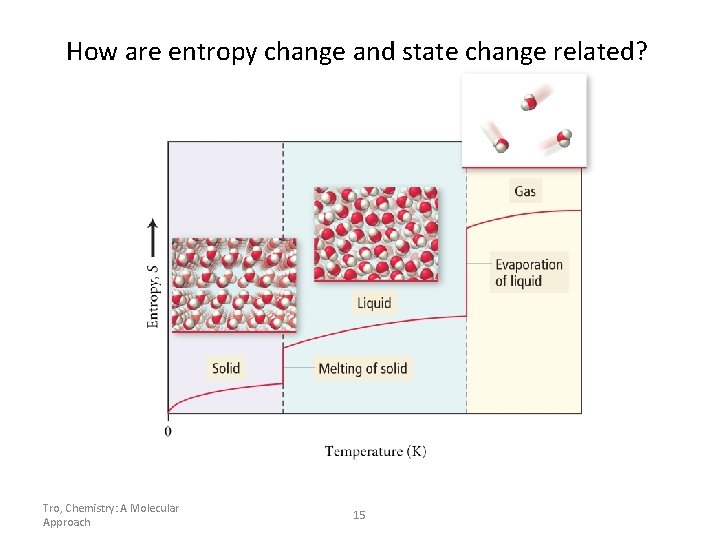
How are entropy change and state change related? Tro, Chemistry: A Molecular Approach 15

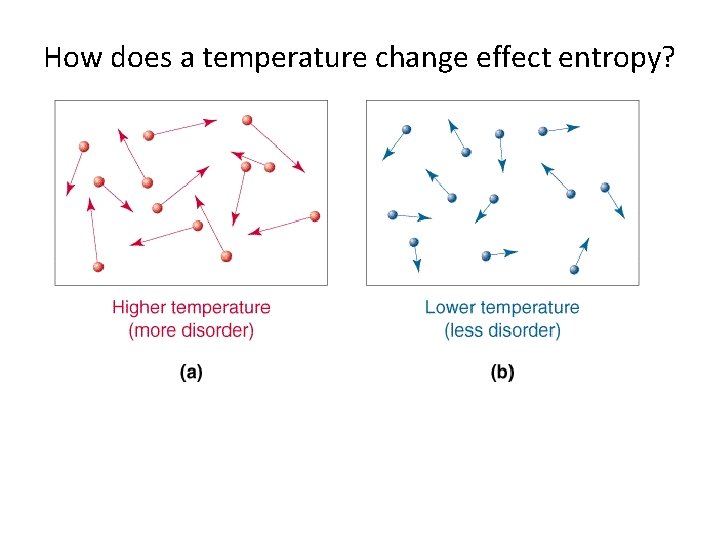
How does a temperature change effect entropy?

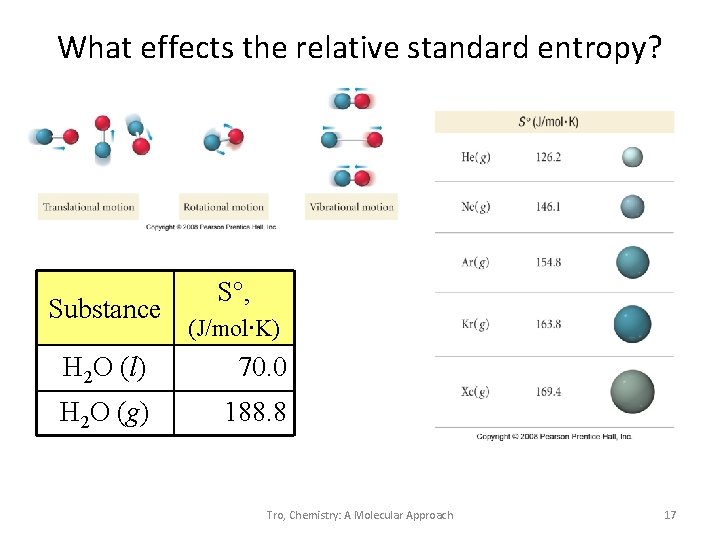
What effects the relative standard entropy? Substance S°, (J/mol∙K) H 2 O (l) 70. 0 H 2 O (g) 188. 8 Tro, Chemistry: A Molecular Approach 17

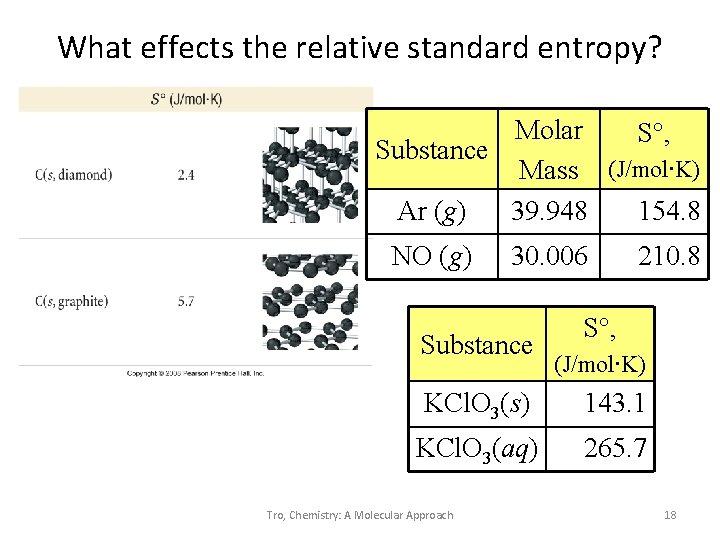
What effects the relative standard entropy? Molar S°, Substance Mass (J/mol∙K) Ar (g) 39. 948 154. 8 NO (g) 30. 006 Substance 210. 8 S°, (J/mol∙K) KCl. O 3(s) 143. 1 KCl. O 3(aq) 265. 7 Tro, Chemistry: A Molecular Approach 18

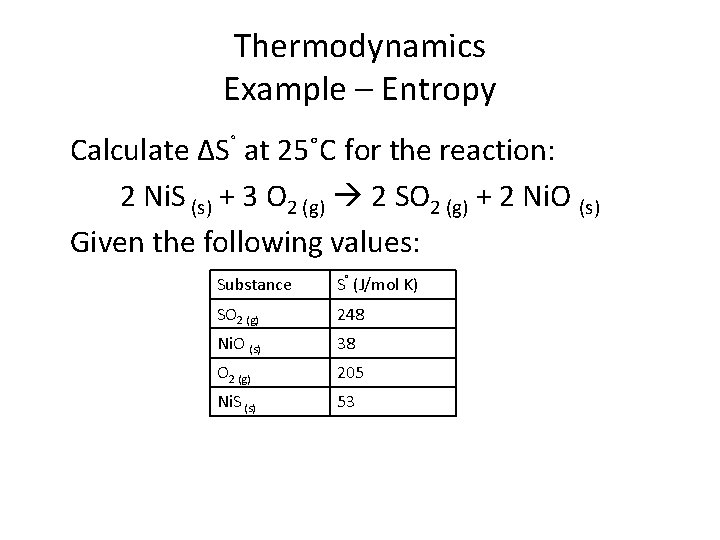
Thermodynamics Example – Entropy Calculate ΔS˚ at 25˚C for the reaction: 2 Ni. S (s) + 3 O 2 (g) 2 SO 2 (g) + 2 Ni. O (s) Given the following values: Substance S˚ (J/mol K) SO 2 (g) 248 Ni. O (s) 38 O 2 (g) 205 Ni. S (s) 53

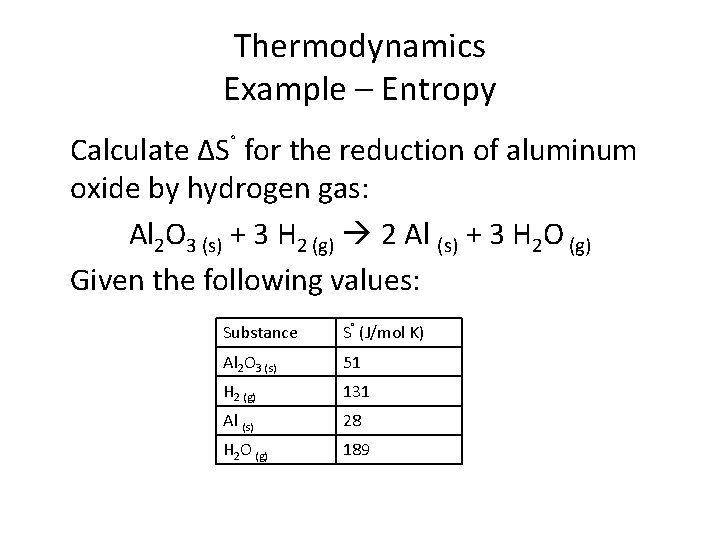
Thermodynamics Example – Entropy Calculate ΔS˚ for the reduction of aluminum oxide by hydrogen gas: Al 2 O 3 (s) + 3 H 2 (g) 2 Al (s) + 3 H 2 O (g) Given the following values: Substance S˚ (J/mol K) Al 2 O 3 (s) 51 H 2 (g) 131 Al (s) 28 H 2 O (g) 189

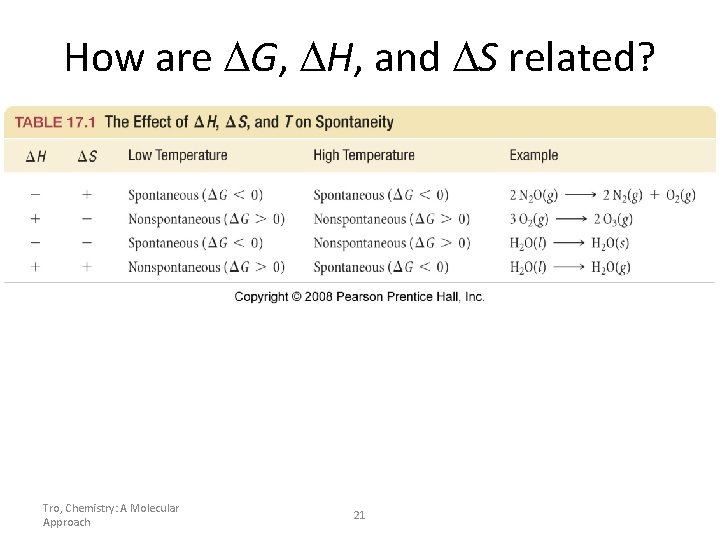
How are G, H, and S related? Tro, Chemistry: A Molecular Approach 21

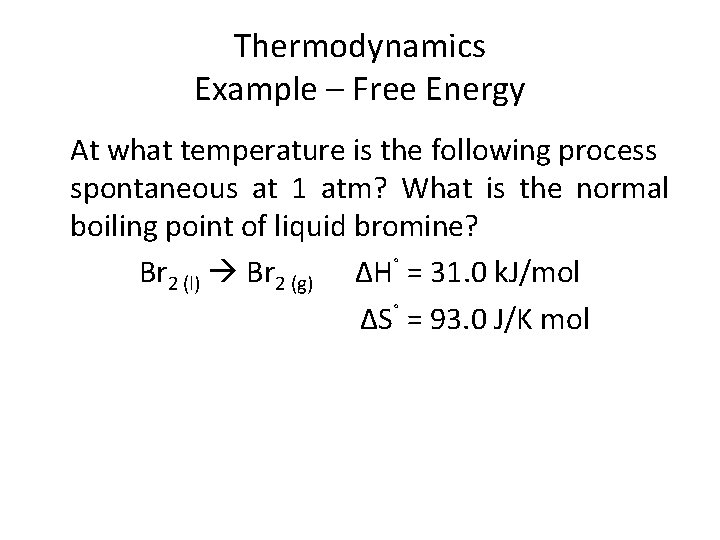
Thermodynamics Example – Free Energy At what temperature is the following process spontaneous at 1 atm? What is the normal boiling point of liquid bromine? Br 2 (l) Br 2 (g) ΔH˚ = 31. 0 k. J/mol ΔS˚ = 93. 0 J/K mol

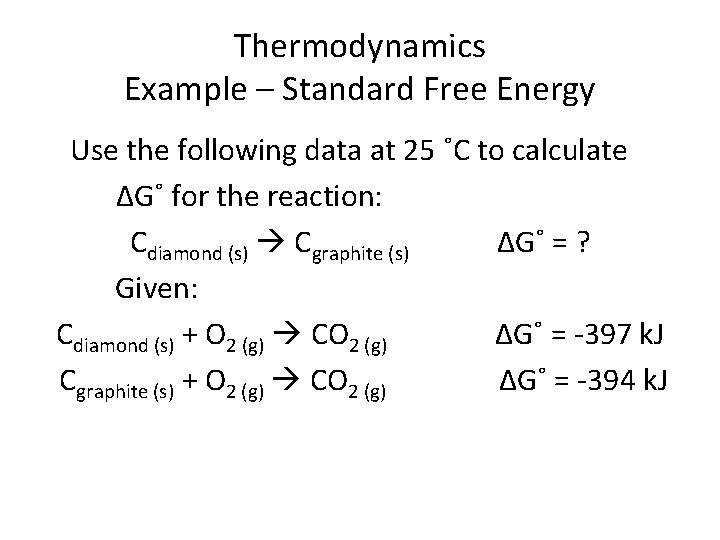
Thermodynamics Example – Standard Free Energy Use the following data at 25 ˚C to calculate ΔG˚ for the reaction: Cdiamond (s) Cgraphite (s) ΔG˚ = ? Given: Cdiamond (s) + O 2 (g) CO 2 (g) ΔG˚ = -397 k. J Cgraphite (s) + O 2 (g) CO 2 (g) ΔG˚ = -394 k. J

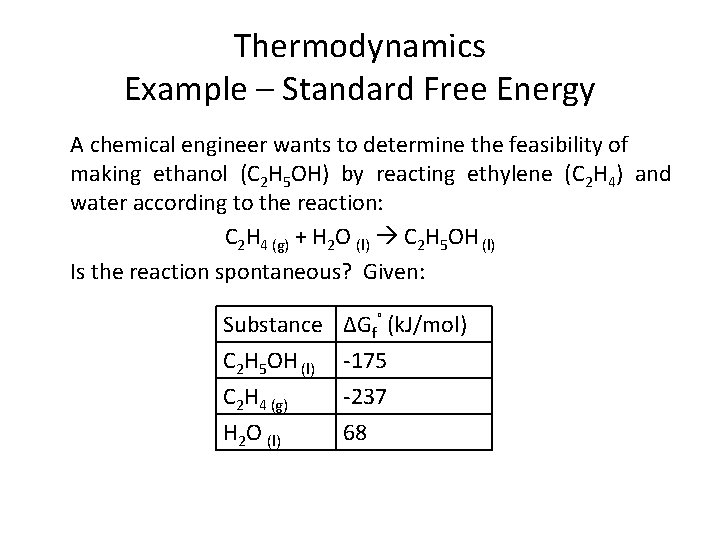
Thermodynamics Example – Standard Free Energy A chemical engineer wants to determine the feasibility of making ethanol (C 2 H 5 OH) by reacting ethylene (C 2 H 4) and water according to the reaction: C 2 H 4 (g) + H 2 O (l) C 2 H 5 OH (l) Is the reaction spontaneous? Given: Substance C 2 H 5 OH (l) C 2 H 4 (g) H 2 O (l) ΔGf˚ (k. J/mol) -175 -237 68

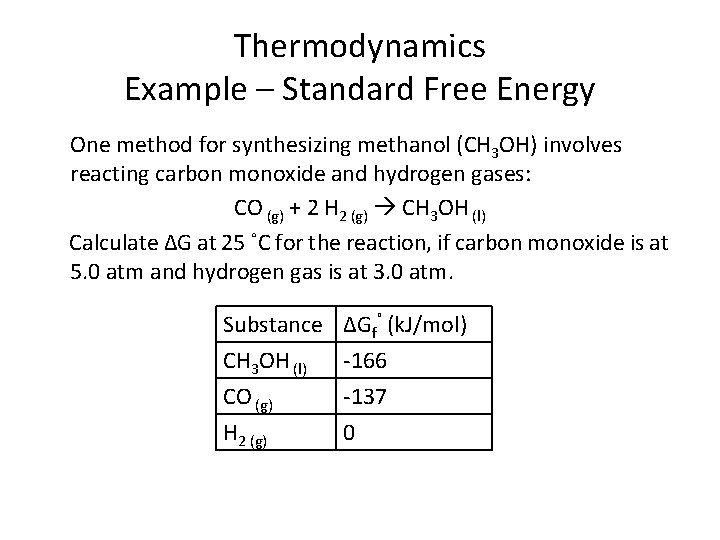
Thermodynamics Example – Standard Free Energy One method for synthesizing methanol (CH 3 OH) involves reacting carbon monoxide and hydrogen gases: CO (g) + 2 H 2 (g) CH 3 OH (l) Calculate ΔG at 25 ˚C for the reaction, if carbon monoxide is at 5. 0 atm and hydrogen gas is at 3. 0 atm. Substance CH 3 OH (l) CO (g) H 2 (g) ΔGf˚ (k. J/mol) -166 -137 0

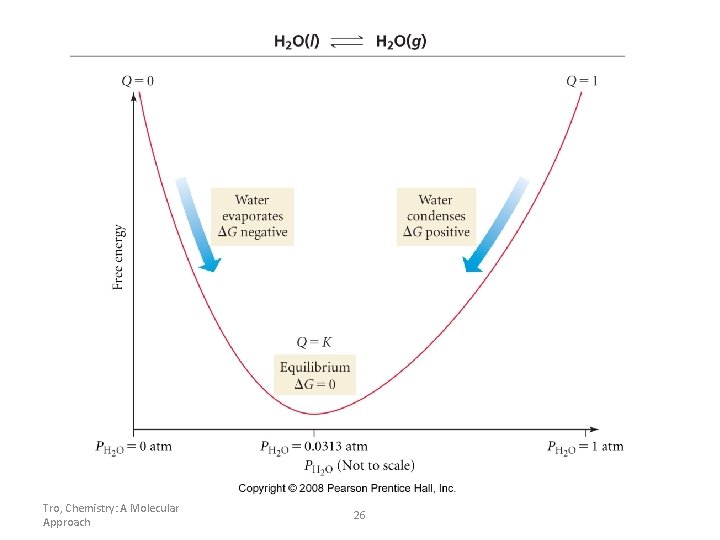
Tro, Chemistry: A Molecular Approach 26

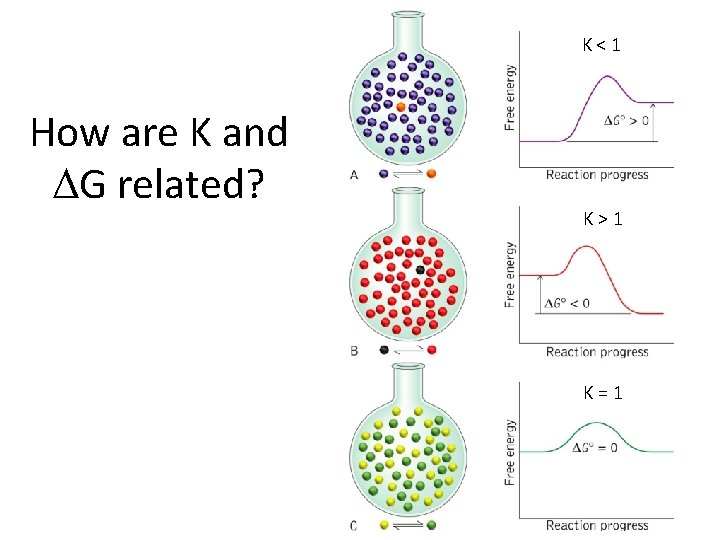
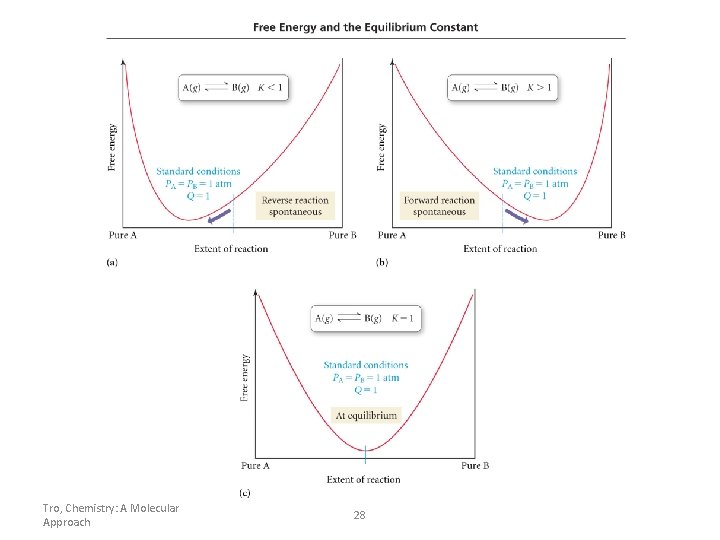
K<1 How are K and G related? K>1 K=1

Tro, Chemistry: A Molecular Approach 28

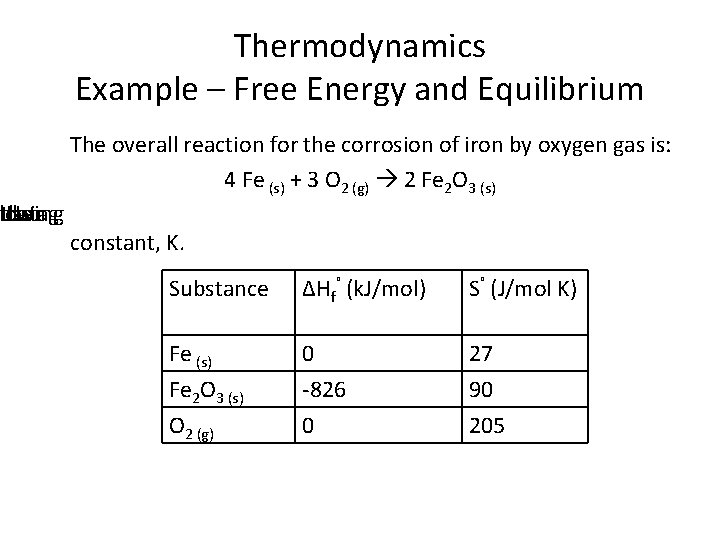
atlowing the data Using Thermodynamics Example – Free Energy and Equilibrium The overall reaction for the corrosion of iron by oxygen gas is: 4 Fe (s) + 3 O 2 (g) 2 Fe 2 O 3 (s) constant, K. Substance ΔHf˚ (k. J/mol) S˚ (J/mol K) Fe (s) Fe 2 O 3 (s) O 2 (g) 0 -826 0 27 90 205

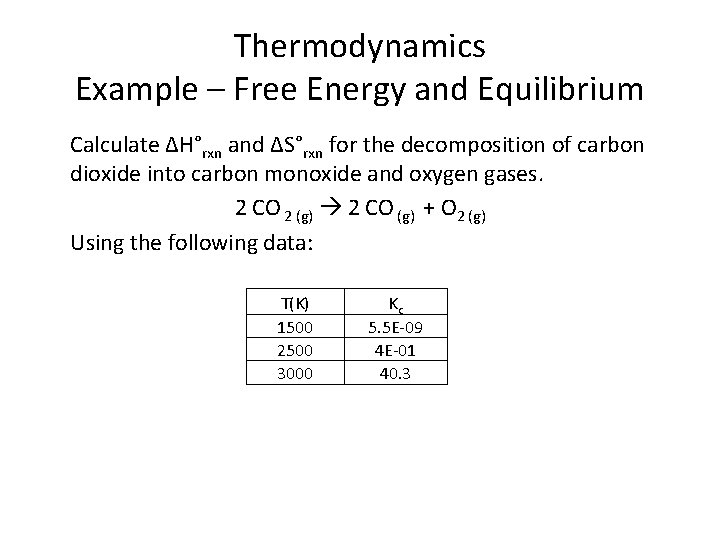
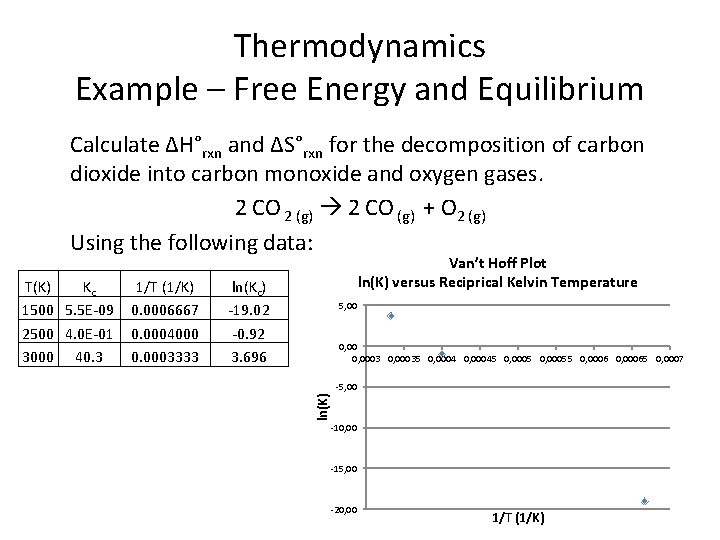
Thermodynamics Example – Free Energy and Equilibrium Calculate ∆H°rxn and ∆S°rxn for the decomposition of carbon dioxide into carbon monoxide and oxygen gases. 2 CO 2 (g) 2 CO (g) + O 2 (g) Using the following data: T(K) 1500 2500 3000 Kc 5. 5 E-09 4 E-01 40. 3

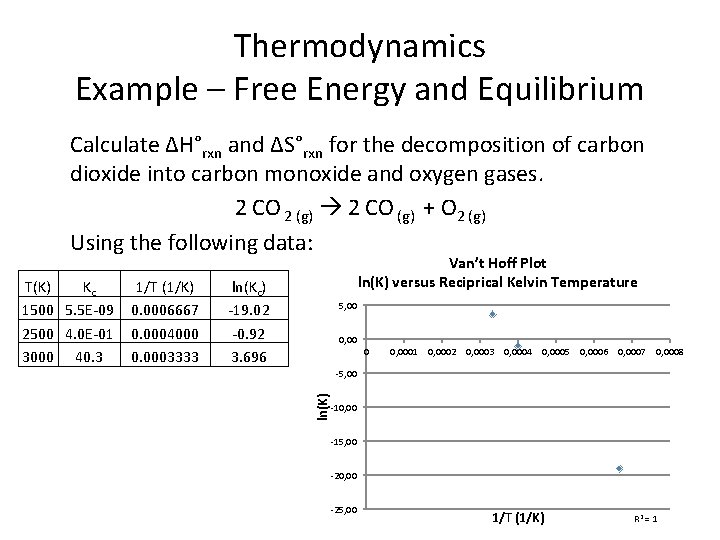
Thermodynamics Example – Free Energy and Equilibrium Calculate ∆H°rxn and ∆S°rxn for the decomposition of carbon dioxide into carbon monoxide and oxygen gases. 2 CO 2 (g) 2 CO (g) + O 2 (g) Using the following data: 1/T (1/K) 0. 0006667 0. 0004000 0. 0003333 ln(Kc) -19. 02 -0. 92 3. 696 5, 00 0 0, 0001 0, 0002 0, 0003 0, 0004 0, 0005 0, 0006 0, 0007 0, 0008 -5, 00 ln(K) T(K) Kc 1500 5. 5 E-09 2500 4. 0 E-01 3000 40. 3 Van’t Hoff Plot ln(K) versus Reciprical Kelvin Temperature -10, 00 -15, 00 -20, 00 -25, 00 1/T (1/K) R 2 = 1

Thermodynamics Example – Free Energy and Equilibrium Calculate ∆H°rxn and ∆S°rxn for the decomposition of carbon dioxide into carbon monoxide and oxygen gases. 2 CO 2 (g) 2 CO (g) + O 2 (g) Using the following data: 1/T (1/K) 0. 0006667 0. 0004000 0. 0003333 ln(Kc) -19. 02 -0. 92 3. 696 5, 00 0, 00035 0, 00045 0, 00055 0, 00065 0, 0007 -5, 00 ln(K) T(K) Kc 1500 5. 5 E-09 2500 4. 0 E-01 3000 40. 3 Van’t Hoff Plot ln(K) versus Reciprical Kelvin Temperature -10, 00 -15, 00 -20, 00 1/T (1/K)

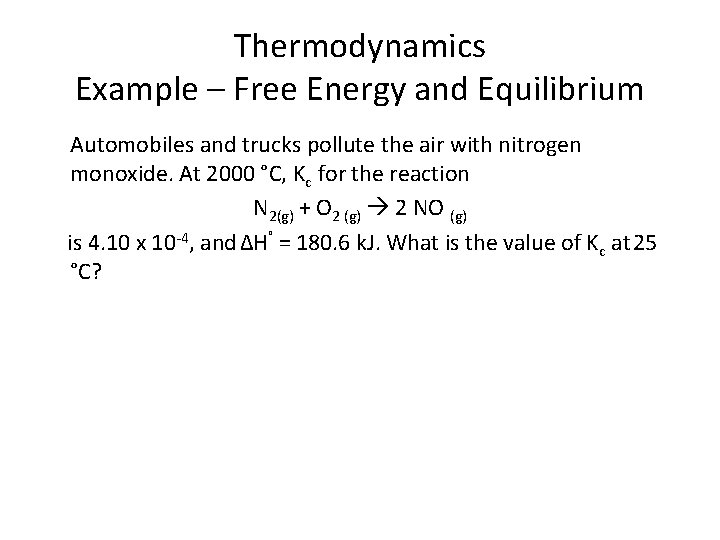
Thermodynamics Example – Free Energy and Equilibrium Automobiles and trucks pollute the air with nitrogen monoxide. At 2000 °C, Kc for the reaction N 2(g) + O 2 (g) 2 NO (g) is 4. 10 x 10 -4, and ΔH˚ = 180. 6 k. J. What is the value of Kc at 25 °C?

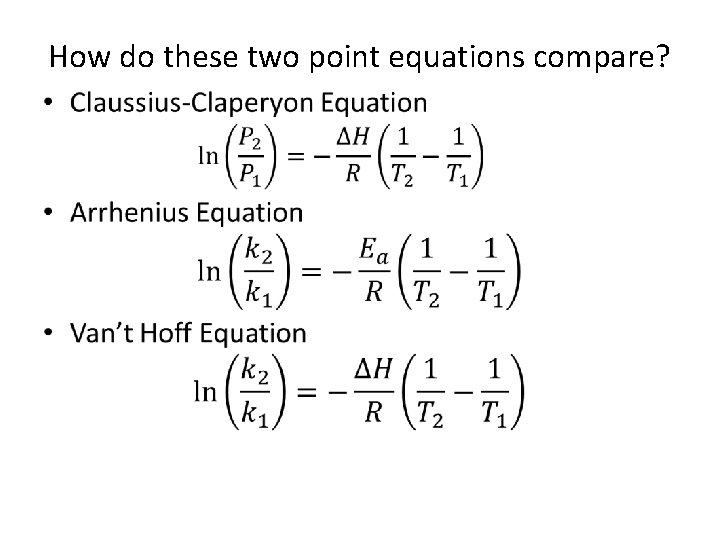
How do these two point equations compare? •

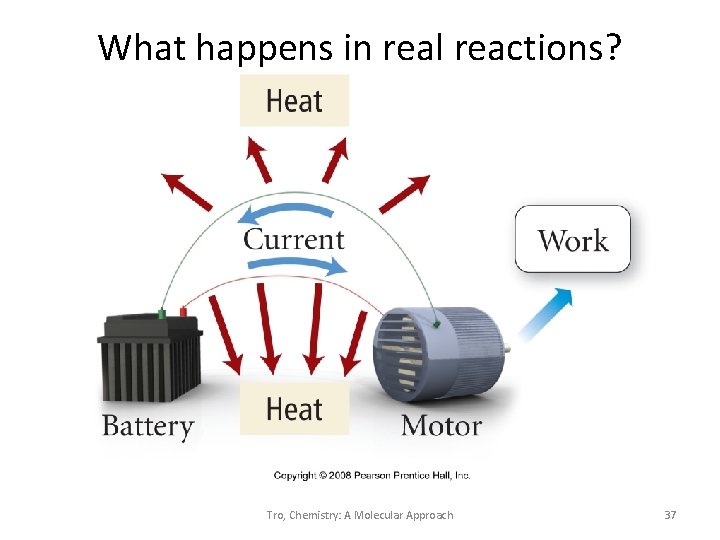
What is the energy tax? • you can’t break even! • to recharge a battery with 100 k. J of useful energy will require more than 100 k. J • every energy transition results in a “loss” of energy – conversion of energy to heat which is “lost” by heating up the surroundings Tro, Chemistry: A Molecular Approach 35

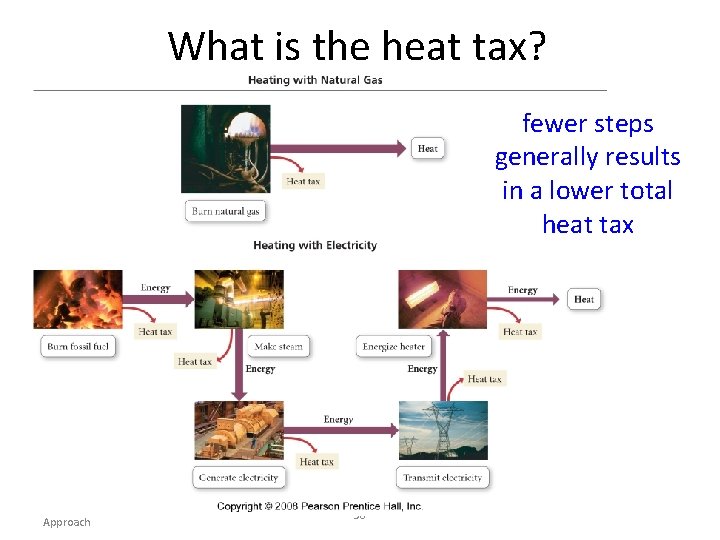
What is the heat tax? fewer steps generally results in a lower total heat tax Tro, Chemistry: A Molecular Approach 36

What happens in real reactions? Tro, Chemistry: A Molecular Approach 37