Chemistry 141 Guidelines for Laboratory Notebooks and Formal

Chemistry 141 Guidelines for Laboratory Notebooks and Formal Laboratory Reports

Lab Books • Initial set up – Get a lab book with prenumbered carbonless copy pages. – Write your name, course, section, and semester on the front of the lab book. – Fill out the title page with your name, etc. – Add the experiment number, title, and pages numbers to the table of contents throughout the semester.

Lab Books • Be sure to write all data in • No writeovers your lab book in black or – 375. 788 g 636 blue ink when you take the data. • No obliterations – The only exception will be – 639. 824 g for data collected by the computer. – Any data recorded on stray pieces of paper will be confiscated! • No not remove pages from your lab book unless you are turning them in for grading. • No Erasing • Cross out once and rewrite. – 4. 932 g – 5. 382 g
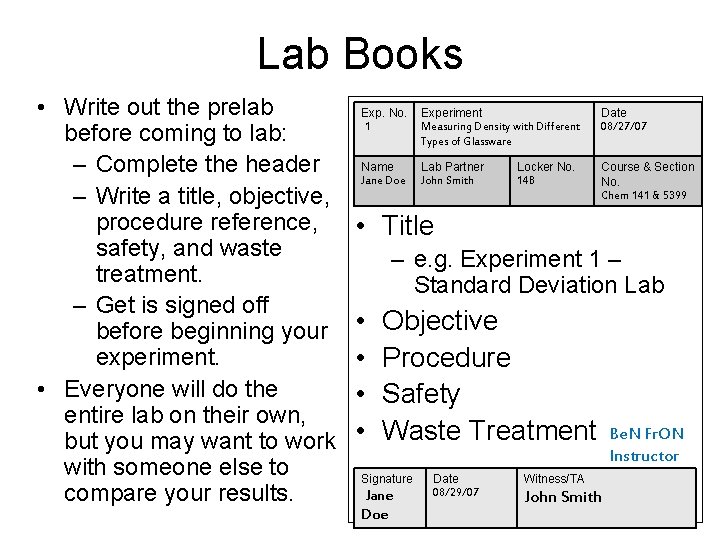
Lab Books • Write out the prelab before coming to lab: – Complete the header – Write a title, objective, procedure reference, safety, and waste treatment. – Get is signed off before beginning your experiment. • Everyone will do the entire lab on their own, but you may want to work with someone else to compare your results. Exp. No. 1 Experiment Measuring Density with Different Types of Glassware Date 08/27/07 Name Jane Doe Lab Partner John Smith Course & Section No. Chem 141 & 5399 Locker No. 14 B • Title – e. g. Experiment 1 – Standard Deviation Lab • • Objective Procedure Safety Waste Treatment Signature Jane Doe Date 08/29/07 Witness/TA John Smith Be. N Fr. ON Instructor
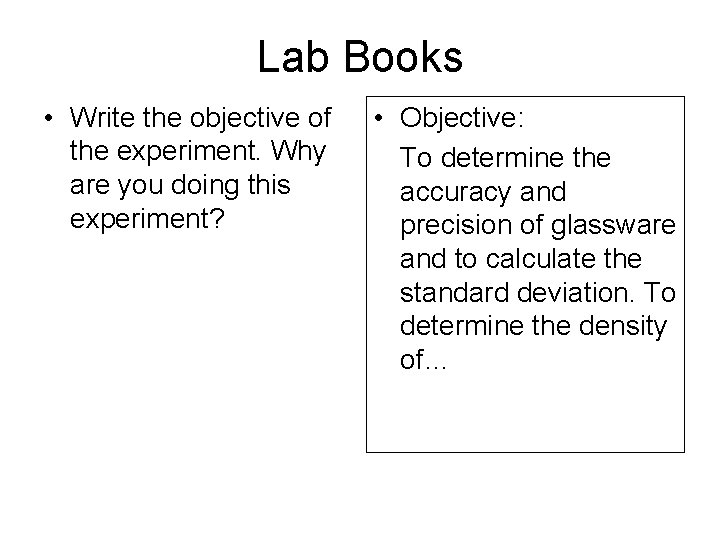
Lab Books • Write the objective of the experiment. Why are you doing this experiment? • Objective: To determine the accuracy and precision of glassware and to calculate the standard deviation. To determine the density of…

Lab Books • If you followed the procedure outlined in the handout provided in lab then – reference the procedure, leave space for changes – note any safety precautions – note the waste treatment Or if you used the Online Lab Manual: Lehman, J. , Olmstead, T. et al (2002). Experiment 2: Measuring Density using different types of Glassware [Electronic version]. Grossmont College Chemistry 141 Laboratory Manual, 5 -10. • Procedure: – Lehman, J. et al (2012). “Experiment 1: Measuring Density with Different Types of Glassware”, Grossmont College, Chemistry 141 Laboratory Manual (6 th Edition, pp 1 -12) El Cajon, California. – Part B Procedure (You will make up your own, but sure to note it here). • Safety: – Wear safety glasses • Waste Treatment: – n/a

Lab Books Data and Observations • This should be a log of what you saw along with any tables of data that may be necessary. – Another student should be able to pick up your lab notebook and repeat your experiment. • There are several days to record your experimental data chose the one that works best for you and the experiment. • Be sure to draw and label the equipment used.

Lab Books Data and Observations • Some options 1. Procedure Reference only • As you complete the experiment write a running log that describes the steps that you are taking to complete the experiment along with your observations, and data. • It is a good idea to write your data tables before you come to lab. 2. Procedure Reference and Procedure Summary • Summarize the procedure and write the data tables before you come to lab: A. Use the left column to write, in our own words, what you think you will do during the experiment. Use the right column to record experimental observations B. Use the whole page to write the procedures with the data tables underneath. Record your experimental observations under the data tables. C. Create a flowchart to organize your experimental workflow.
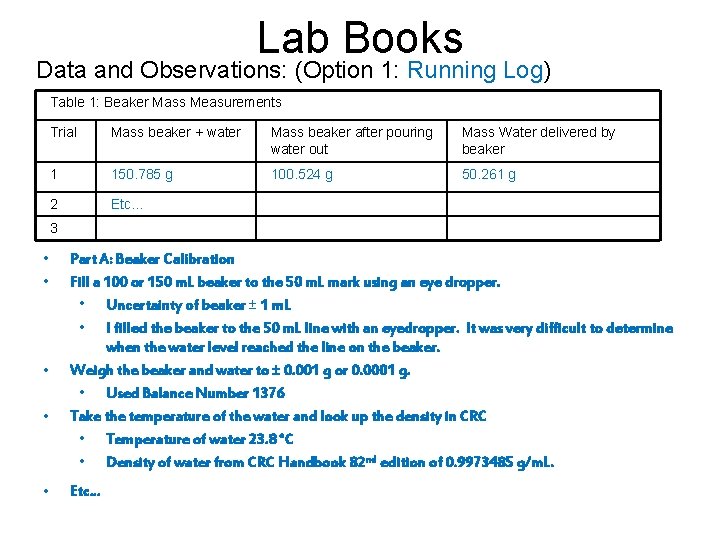
Lab Books Data and Observations: (Option 1: Running Log) Table 1: Beaker Mass Measurements Trial Mass beaker + water Mass beaker after pouring water out Mass Water delivered by beaker 1 150. 785 g 100. 524 g 50. 261 g 2 Etc… 3 • • • Part A: Beaker Calibration Fill a 100 or 150 m. L beaker to the 50 m. L mark using an eye dropper. • Uncertainty of beaker ± 1 m. L • I filled the beaker to the 50 m. L line with an eyedropper. It was very difficult to determine when the water level reached the line on the beaker. Weigh the beaker and water to ± 0. 001 g or 0. 0001 g. • Used Balance Number 1376 Take the temperature of the water and look up the density in CRC • Temperature of water 23. 8 °C • Density of water from CRC Handbook 82 nd edition of 0. 9973485 g/m. L. Etc…
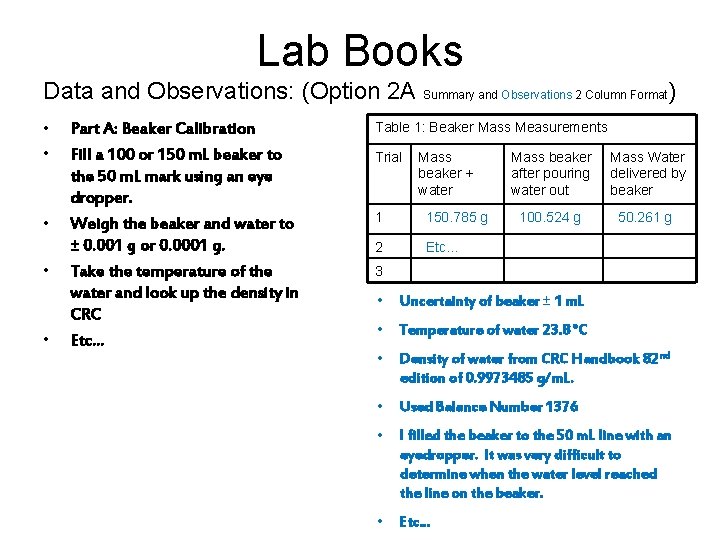
Lab Books Data and Observations: (Option 2 A Summary and Observations 2 Column Format) • • • Part A: Beaker Calibration Fill a 100 or 150 m. L beaker to the 50 m. L mark using an eye dropper. Weigh the beaker and water to ± 0. 001 g or 0. 0001 g. Take the temperature of the water and look up the density in CRC Etc… Table 1: Beaker Mass Measurements Trial Mass beaker + water 1 150. 785 g 2 Etc… Mass beaker after pouring water out Mass Water delivered by beaker 100. 524 g 50. 261 g 3 • Uncertainty of beaker ± 1 m. L • Temperature of water 23. 8 °C • Density of water from CRC Handbook 82 nd edition of 0. 9973485 g/m. L. • Used Balance Number 1376 • I filled the beaker to the 50 m. L line with an eyedropper. It was very difficult to determine when the water level reached the line on the beaker. • Etc…
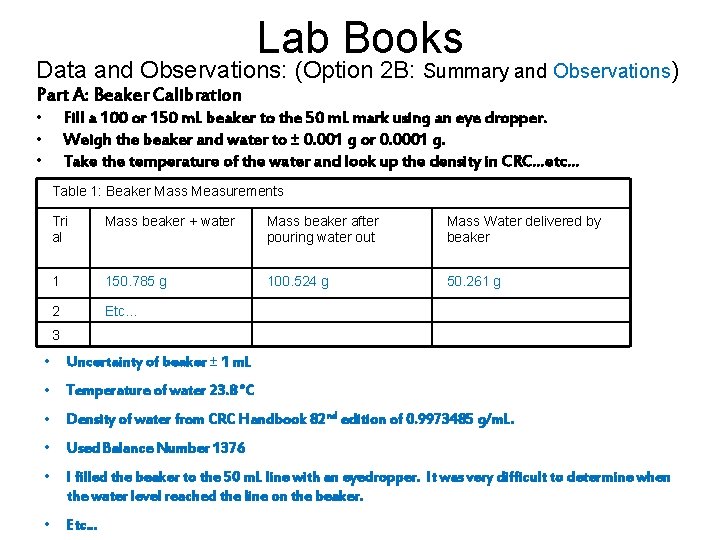
Lab Books Data and Observations: (Option 2 B: Summary and Observations) Part A: Beaker Calibration • • • Fill a 100 or 150 m. L beaker to the 50 m. L mark using an eye dropper. Weigh the beaker and water to ± 0. 001 g or 0. 0001 g. Take the temperature of the water and look up the density in CRC…etc… Table 1: Beaker Mass Measurements Tri al Mass beaker + water Mass beaker after pouring water out Mass Water delivered by beaker 1 150. 785 g 100. 524 g 50. 261 g 2 Etc… 3 • Uncertainty of beaker ± 1 m. L • Temperature of water 23. 8 °C • Density of water from CRC Handbook 82 nd edition of 0. 9973485 g/m. L. • Used Balance Number 1376 • I filled the beaker to the 50 m. L line with an eyedropper. It was very difficult to determine when the water level reached the line on the beaker. • Etc…

Lab Books Exp. No. 7 Experiment Copper Reactions Date 09/15/07 • At the end of the lab Name Lab Partner Locker No. Course & Section 14 B No. period, sign and date Jane Doe n/a Chem 141 & 5399 your lab book after your last data entry • Copper sulfide precipitate was filtered and left in lab drawer to and get an instructor dry. The precipitate was a very stamp. fine black powder. Be. N Fr. ON Instructor Signature Jane Doe Date 09/15/07 Witness/TA John Smith

Lab Reports • Typewritten reports documenting your experimental results. Some reports will be abbreviated as noted in write-up instructions. • Be sure to clearly label each section! • • • Title Page Objective Introduction Procedure Results and Calculations • Discussion • Conclusion • Questions

Lab Reports: Title Page • This will identify the experiment, you, the course, your class, your instructor, and the date(s) the experiment was performed. Very important so I can keep track of what you are handing in! Standard Deviation Experiment Fun with Glassware! Jane Doe Lab Partner: John Smith Chemistry 141 Section 5399 Instructor: Be. N Fr. ON August 29, 2007 completed September 7, 2007 turned in
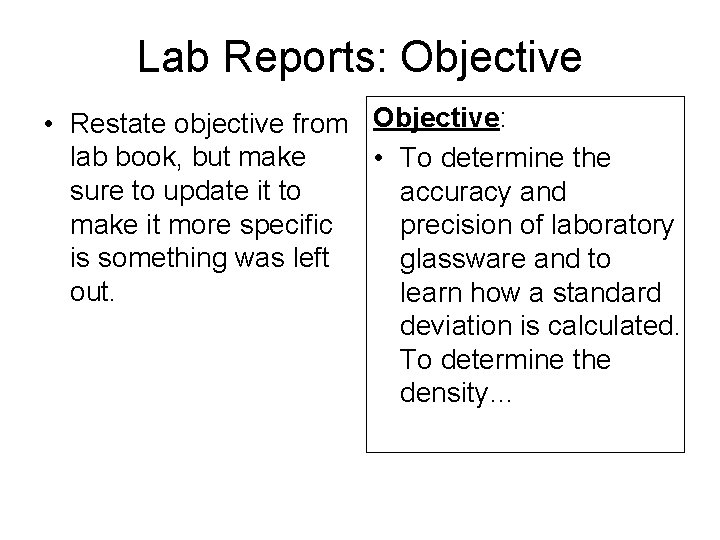
Lab Reports: Objective • Restate objective from Objective: lab book, but make • To determine the sure to update it to accuracy and make it more specific precision of laboratory is something was left glassware and to out. learn how a standard deviation is calculated. To determine the density…

Lab Reports: Introduction • This section tells a little bit about theory of the experiment and how it will be done. • Be sure to give each equation and/or reaction it’s own line and label is eq. 1, eq. 2, rxn 1, rxn 2, etc…so that you can refer back to then by number later in your report. Introduction: • When doing scientific experiments it is always necessary to minimize the error where possible. Unfortunately, however, it is impossible to completely avoid error. In experiments there are several types of error. They are random error, systematic error, and gross error. Random error is---

Lab Reports: Procedure • Reference the procedure used and note any deviations from the published procedure. Or if you used the Online Lab Manual: Lehman, J. , Olmstead, T. et al (2002). Experiment 2: Measuring Density using different types of Glassware [Electronic version]. Grossmont College Chemistry 141 Laboratory Manual, 5 -10. Procedure • Followed procedure from handout: – Lehman, J. et al (2012). “Experiment 1: Measuring Density with Different Types of Glassware”, Grossmont College, Chemistry 141 Laboratory Manual (6 th Edition, pp 1 -12) El Cajon, California. – Part B: Density of Coke and Diet Coke 1. Selected most accurate and precise piece of glassware the… 2. … • Note any changes – e. g. In this experiment the liquid used for the density determinations was an isopropanol/water mixture.
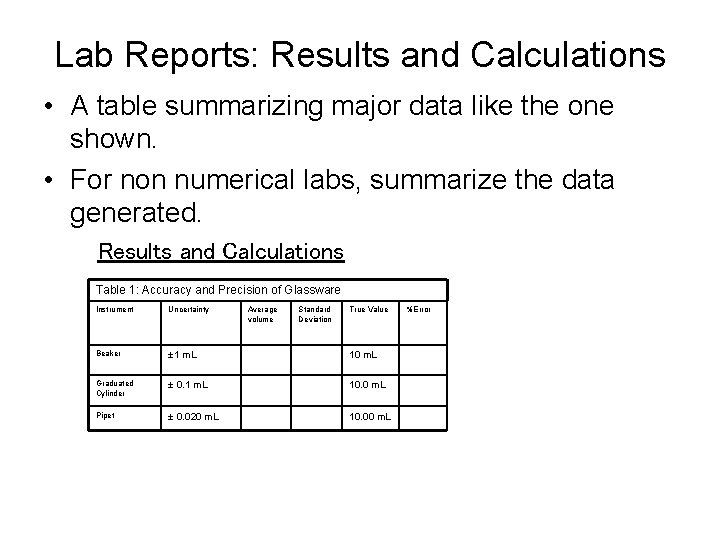
Lab Reports: Results and Calculations • A table summarizing major data like the one shown. • For non numerical labs, summarize the data generated. Results and Calculations Table 1: Accuracy and Precision of Glassware Instrument Uncertainty Average volume Standard Deviation True Value Beaker ± 1 m. L 10 m. L Graduated Cylinder ± 0. 1 m. L 10. 0 m. L Pipet ± 0. 020 m. L 10. 00 m. L %Error
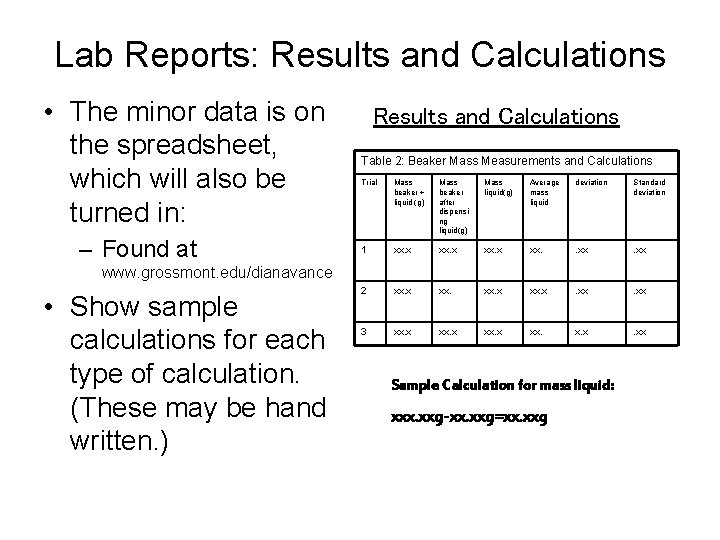
Lab Reports: Results and Calculations • The minor data is on the spreadsheet, which will also be turned in: – Found at Results and Calculations Table 2: Beaker Mass Measurements and Calculations Trial Mass beaker + liquid (g) Mass beaker after dispensi ng liquid(g) Mass liquid(g) Average mass liquid deviation Standard deviation 1 xx. x xx. . xx 2 xx. x . xx 3 xx. x xx. x. x . xx www. grossmont. edu/dianavance • Show sample calculations for each type of calculation. (These may be hand written. ) Sample Calculation for mass liquid: xxx. xxg-xx. xxg=xx. xxg
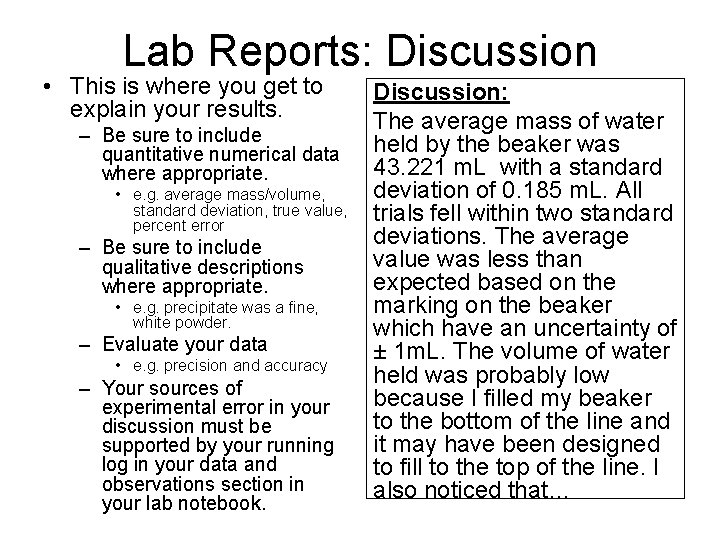
Lab Reports: Discussion • This is where you get to explain your results. – Be sure to include quantitative numerical data where appropriate. • e. g. average mass/volume, standard deviation, true value, percent error – Be sure to include qualitative descriptions where appropriate. • e. g. precipitate was a fine, white powder. – Evaluate your data • e. g. precision and accuracy – Your sources of experimental error in your discussion must be supported by your running log in your data and observations section in your lab notebook. Discussion: The average mass of water held by the beaker was 43. 221 m. L with a standard deviation of 0. 185 m. L. All trials fell within two standard deviations. The average value was less than expected based on the marking on the beaker which have an uncertainty of ± 1 m. L. The volume of water held was probably low because I filled my beaker to the bottom of the line and it may have been designed to fill to the top of the line. I also noticed that…
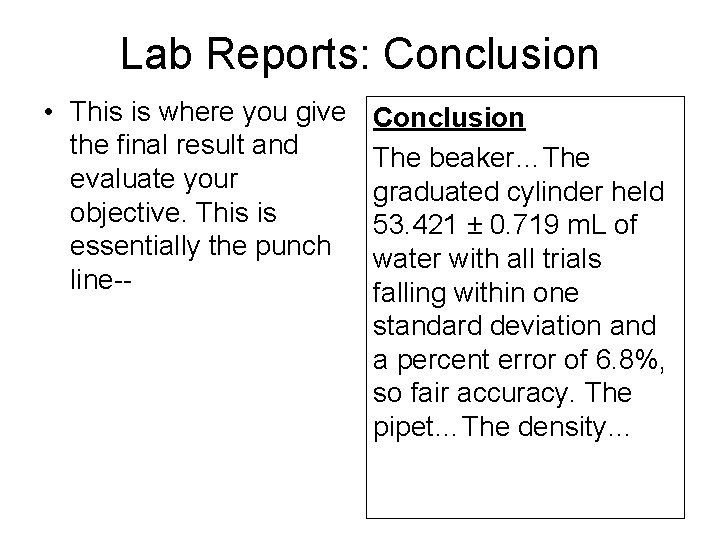
Lab Reports: Conclusion • This is where you give the final result and evaluate your objective. This is essentially the punch line-- Conclusion The beaker…The graduated cylinder held 53. 421 ± 0. 719 m. L of water with all trials falling within one standard deviation and a percent error of 6. 8%, so fair accuracy. The pipet…The density…

Lab Reports: Questions • Answer any questions posed in the lab here or on the sheet(s) provided in the lab manual.
- Slides: 22