Chemistry 139 General Chemistry Prep Atoms Molecules and





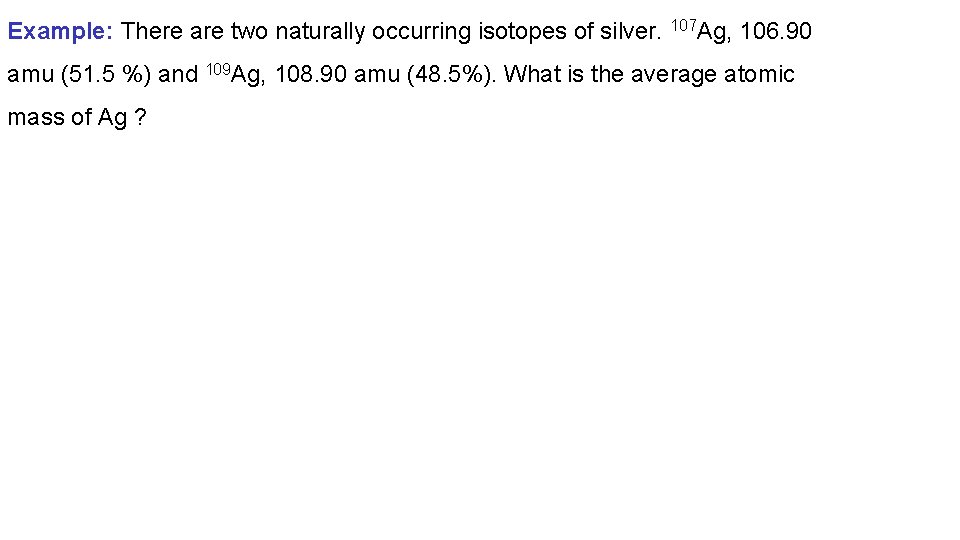












- Slides: 30

Chemistry& 139 General Chemistry Prep

Atoms, Molecules and Subatomic particles 5. 1 The Atom 5. 2 The Molecule 5. 3 Natural and Synthetic Compounds 5. 4 Chemical Formulas 5. 5 Subatomic Particles: Protons, Neutrons and Electrons 5. 6 Atomic Number and Mass Number 5. 7 Isotopes 5. 8 Atomic Masses 5. 9 Evidence Supporting the Existence and Arrangement of Subatomic Particles

Atom Molecules and Subatomic Particles 5. 8 Atomic Masses

The particles that make up atoms and atoms themselves have very small masses when grams are used to express their mass. To get around this problem chemist use a relative mass scale based on the “atomic mass unit” to express the mass of atoms and subatomic particles.

Chemists assigned a mass of 12 atomic mass units (u or sometimes amu) to a 612 C atom. This allows the relative atomic mass for any other atom to be computed as either a multiple or fraction of this number. i. e. 1 amu = 1/12 th the mass of a “carbon-12” atom

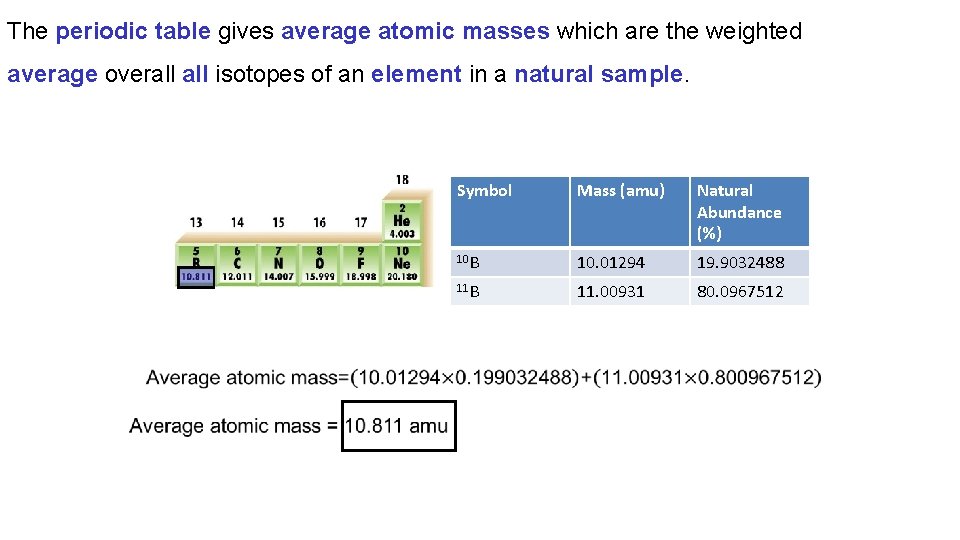
The periodic table gives average atomic masses which are the weighted average overall isotopes of an element in a natural sample. Symbol Mass (amu) Natural Abundance (%) 10 B 10. 01294 19. 9032488 11 B 11. 00931 80. 0967512

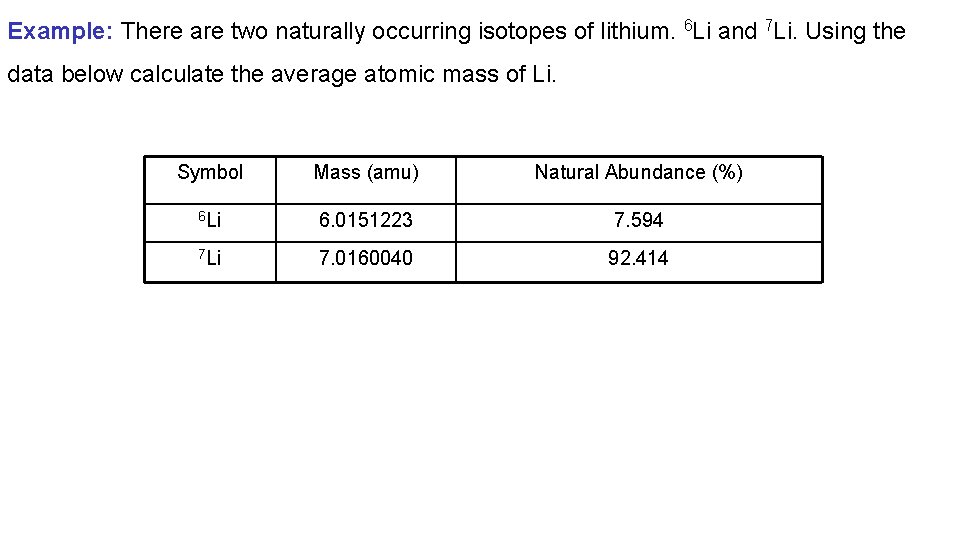
Example: There are two naturally occurring isotopes of lithium. 6 Li and 7 Li. Using the data below calculate the average atomic mass of Li. Symbol Mass (amu) Natural Abundance (%) 6 Li 6. 0151223 7. 594 7 Li 7. 0160040 92. 414
Example: There are two naturally occurring isotopes of silver. 107 Ag, 106. 90 amu (51. 5 %) and 109 Ag, 108. 90 amu (48. 5%). What is the average atomic mass of Ag ?

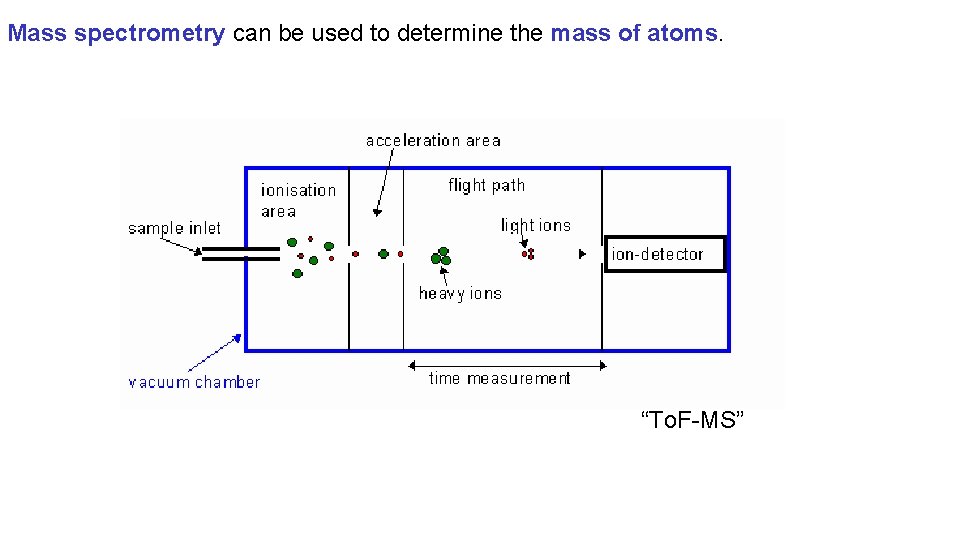
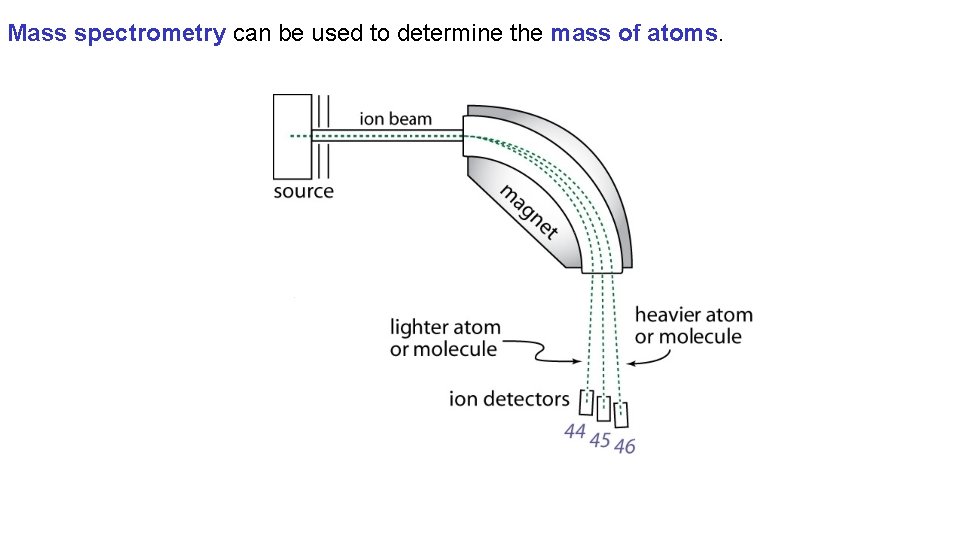
Mass spectrometry can be used to determine the mass of atoms. “To. F-MS”

Mass spectrometry can be used to determine the mass of atoms.
Example: There are two naturally occurring isotopes of silver. 107 Ag, 106. 90 amu (51. 5 %) and 109 Ag, 108. 90 amu (48. 5%). What is the average atomic mass of Ag ?

Atom Molecules and Subatomic Particles 5. 8 Atomic Masses Isotopes of an element have the same number of protons but differing numbers of neutrons. The atomic masses listed in the periodic table are weighted averages of the masses of the isotopes occurring in a natural sample.

Atom Molecules and Subatomic Particles 5. 9 Evidence Supporting the Existence and Arrangement of Subatomic Particles

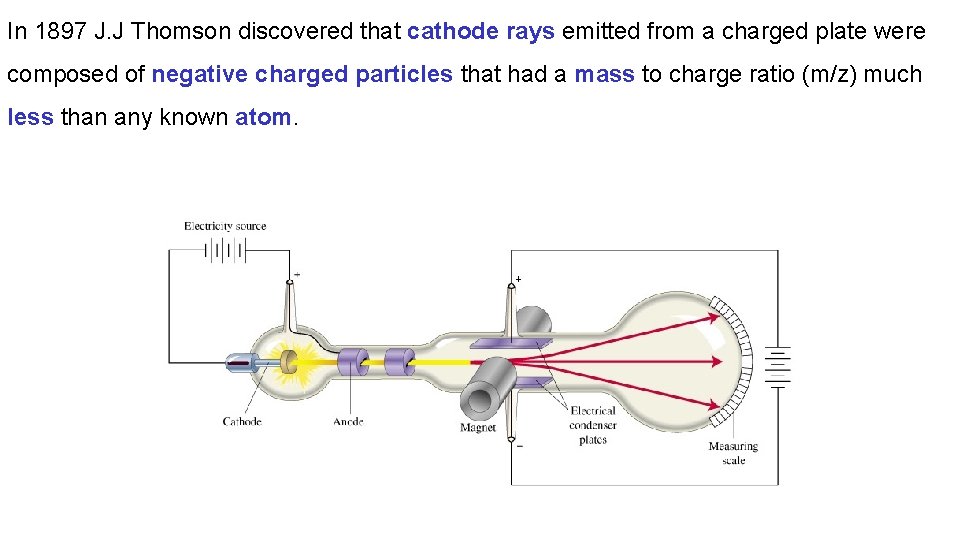
In 1897 J. J Thomson discovered that cathode rays emitted from a charged plate were composed of negative charged particles that had a mass to charge ratio (m/z) much less than any known atom.

It was proposed that these “rays” were subatomic particles. • We now call these particles electrons and often give them the symbol e- or e. The observation of subatomic particles was in violation of Dalton’s first postulate. A new atomic theory was required.

It was proposed that these “rays” were subatomic particles. • We now call these particles electrons and often give them the symbol e- or e. The observation of subatomic particles was in violation of Dalton’s first postulate. A new atomic theory was required.

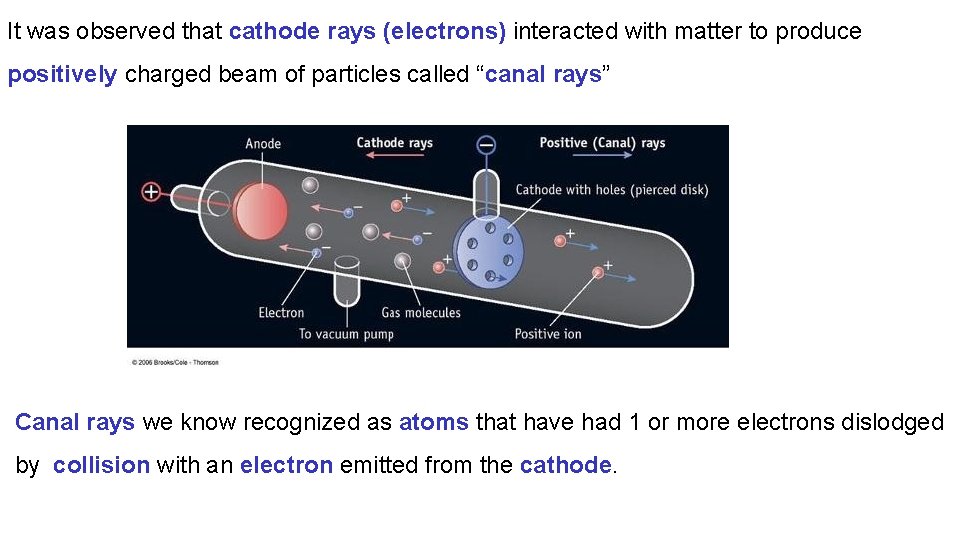
It was observed that cathode rays (electrons) interacted with matter to produce positively charged beam of particles called “canal rays” Canal rays we know recognized as atoms that have had 1 or more electrons dislodged by collision with an electron emitted from the cathode.

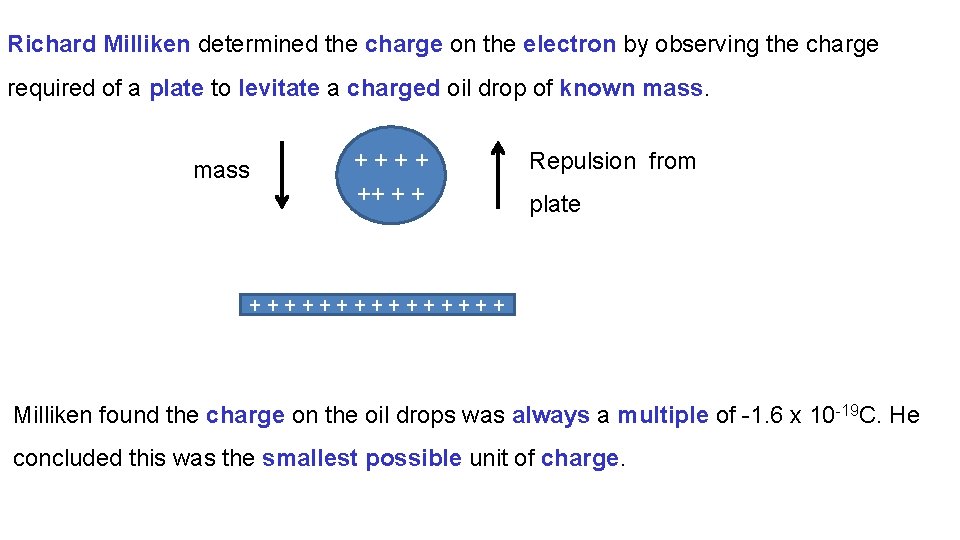
Richard Milliken determined the charge on the electron by observing the charge required of a plate to levitate a charged oil drop of known mass ++++ ++ + + Repulsion from plate ++++++++ Milliken found the charge on the oil drops was always a multiple of -1. 6 x 10 -19 C. He concluded this was the smallest possible unit of charge.

Using Thomson’s data he determined the mass of the electron. • 9. 1 × 10 -28 grams • < 1/1000 th of the lightest known atom The first subatomic particle had been completed characterized. • Are there more subatomic particles? • What balances out the charge to give a neutral atom? • What makes up most of the mass of the atom?

These results led Thomson to propose the “Plum Pudding” model of the atom. In this model the light, negatively charged electrons are embedded in a “sphere of positive charge” to produce a neutral atom. If Thomson lived in the 21 st century he might have called his model the chocchip muffin model.

Henri Becquerel, Pierre and Marie Curie and Ernest Rutherford, discovered that some atoms spontaneously emit particles. These particles were of two types: • β- particles (high energy electrons) • α-particles

-particles are positively charged particles. • They have twice the charge of an electron • Four times the mass of a hydrogen atom. Ernest Rutherford was interested in studying how -particles interacted with matter.

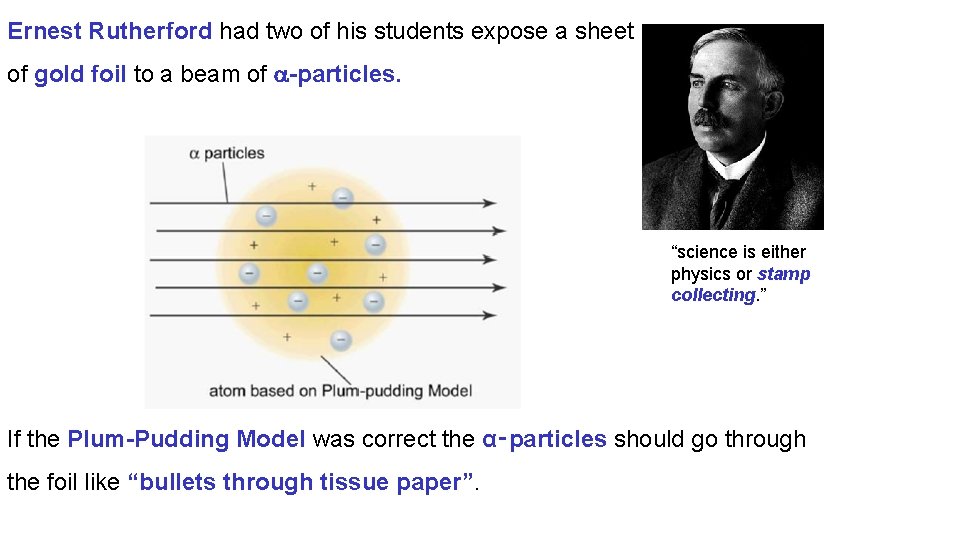
Ernest Rutherford had two of his students expose a sheet of gold foil to a beam of -particles. “science is either physics or stamp collecting. ” If the Plum-Pudding Model was correct the α‑particles should go through the foil like “bullets through tissue paper”.

Geiger-Marsden experiment Most of the α-particles went straight through, but some were deflected or even bounced back!

This like if you were to fired a machine gun at a piece of tissue and occasionally a bullet bounced back and hit you. The only way Rutherford could satisfactorily explain the results of this experiment was as follows: • As most -particles pass through the foil • Atoms must be mostly empty space • As some α-particles are deflected or bounce back • Atoms must contain a positively charged nucleus containing nearly all the mass of the atom.

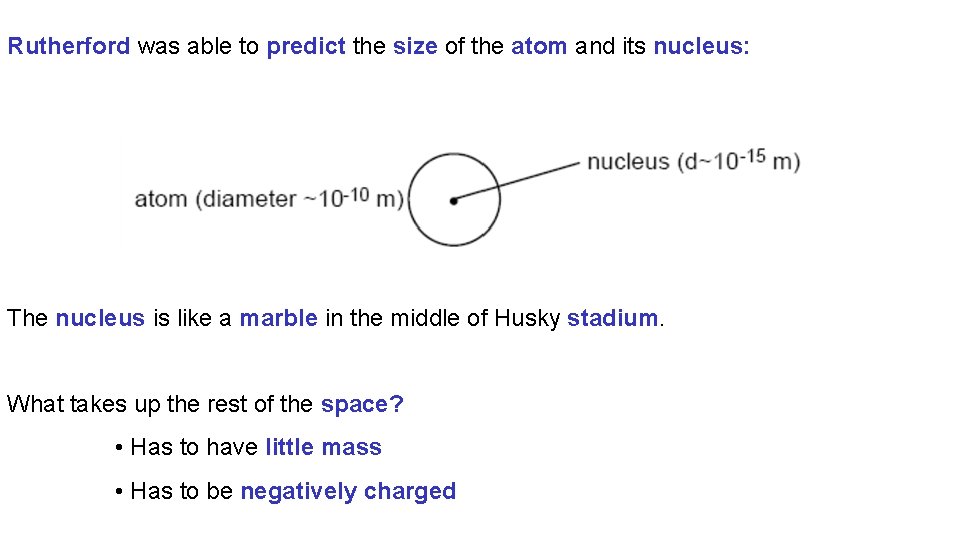
Rutherford was able to predict the size of the atom and its nucleus: The nucleus is like a marble in the middle of Husky stadium. What takes up the rest of the space? • Has to have little mass • Has to be negatively charged

Rutherford proposed that the positive charge of the nucleus was a result of positive charged particles called protons. He proposed that the small negatively charged electrons moved around the nucleus at a great distance from it like planets around a star. However, Rutherford’s model could not account for all the mass of the nucleus.

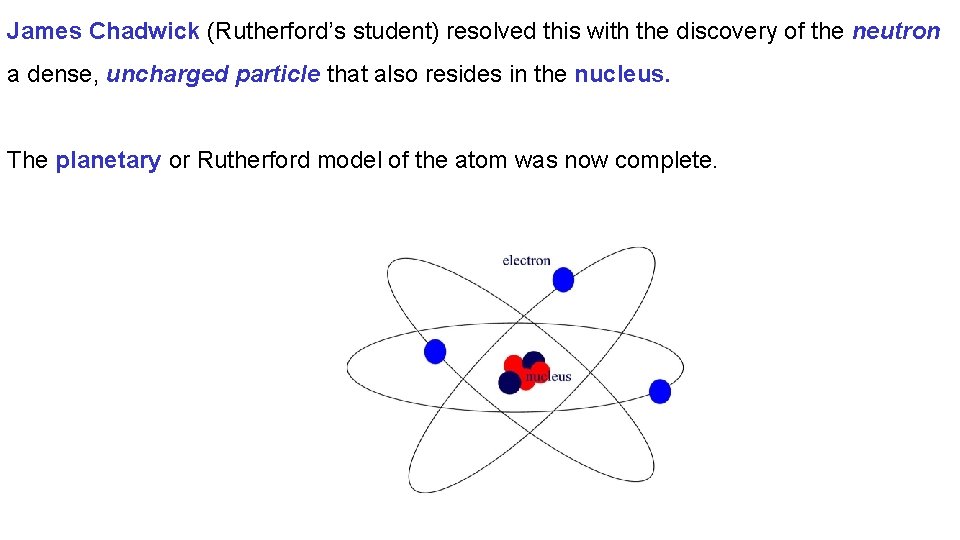
James Chadwick (Rutherford’s student) resolved this with the discovery of the neutron a dense, uncharged particle that also resides in the nucleus. The planetary or Rutherford model of the atom was now complete.

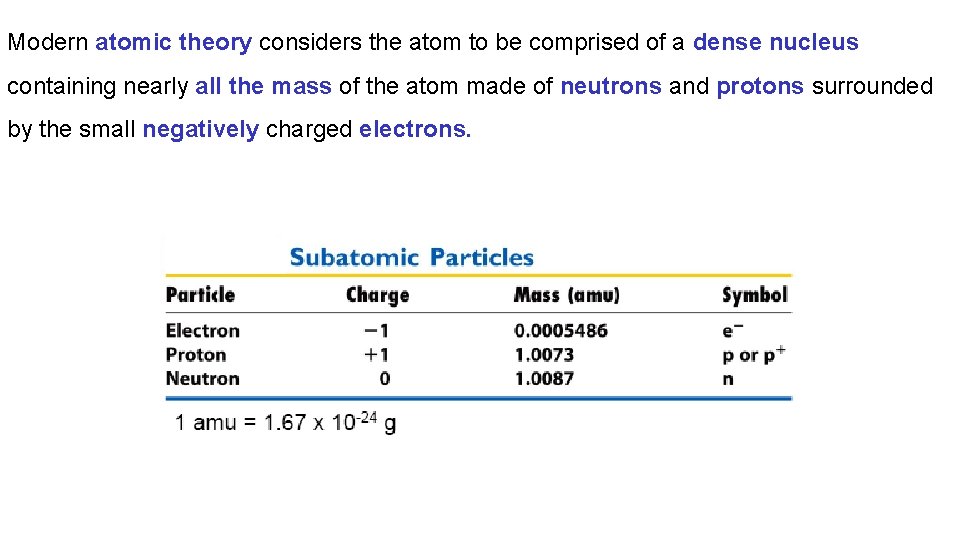
Modern atomic theory considers the atom to be comprised of a dense nucleus containing nearly all the mass of the atom made of neutrons and protons surrounded by the small negatively charged electrons.

Atom Molecules and Subatomic Particles 5. 9 Evidence Supporting the Existence and Arrangement of Subatomic Particles Discharge tube experiments by J. J Thomson resulted in identification of the electron. The formation of positive ions was observed in gas discharge experiments by Eugen Goldstein. Millikan's oil drop experiment provided characterization of the electrons charge and mass. This led Thomson to propose the “plum pudding model” of the atom. Ernest Rutherford’s gold foil experiment resulted in discovery of the nucleus of the atom and led to the Rutherford or planetary model of the atom.
 Relationship between atoms and molecules
Relationship between atoms and molecules Mixture of elements
Mixture of elements 3bacl2 counting atoms
3bacl2 counting atoms Ion chapter 11
Ion chapter 11 Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms ions and molecules
Atoms ions and molecules Atoms ions and molecules
Atoms ions and molecules States that atoms ions and molecules must collide to react
States that atoms ions and molecules must collide to react Chapter 2 atoms molecules and ions
Chapter 2 atoms molecules and ions Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Atoms combine to form
Atoms combine to form Compared to atoms of metals, atoms of nonmetals generally
Compared to atoms of metals, atoms of nonmetals generally Chemsheets as 1017 answers
Chemsheets as 1017 answers Chemistry molecules
Chemistry molecules Vsepr theory
Vsepr theory Shapes of molecules a level
Shapes of molecules a level A level chemistry shapes of molecules
A level chemistry shapes of molecules Ap chemistry electronic structure of atoms
Ap chemistry electronic structure of atoms Planos en cinematografia
Planos en cinematografia Where did general lee surrender to general grant?
Where did general lee surrender to general grant? Jacob's ladder wound closure
Jacob's ladder wound closure Before the statue of liberty toefl
Before the statue of liberty toefl Classify the following unbalanced chemical equations
Classify the following unbalanced chemical equations Site:slidetodoc.com
Site:slidetodoc.com Absolute configuration
Absolute configuration Polar and nonpolar similarities
Polar and nonpolar similarities When a natural disaster destroys a stable ecosystem
When a natural disaster destroys a stable ecosystem Shape and polarity
Shape and polarity Non polar molecules that include fats oils and cholesterol
Non polar molecules that include fats oils and cholesterol