CHEMISTRY 132 SUPPLEMENTAL INSTRUCTION SEPTEMBER 17 TH 2018

CHEMISTRY 132 SUPPLEMENTAL INSTRUCTION SEPTEMBER 17 TH, 2018

HOUSEKEEPING • SI Session Times: • Monday: 7: 30 -9: 00 pm in Dow 107 • Wednesday: 7: 30 -9: 00 pm in Dow 107 • Office Hours: Monday: 11: 00 -12: 00 pm in Ronan 250 • Exam is September 25 th! • Contact Information: • idoni 1 le@cmich. edu • Reminder to sign in for every session

OPENING QUIZ • The density of a 30. 0 mass % solution of sulfuric acid (H 2 SO 4) in water is 1. 1783 g/m. L at 25. 0°C (98. 1 g atomic mass). What is the molarity of the solution? • Assume you have a 12. 5% mass percent solution of Li. Cl in water. What mass of solution in grams contains 3. 3 grams of Li. Cl? • What is the unit concentration for molality?

UNITS OF CONCENTRATION

CHAPTER 11. 4 – FACTORS AFFECTING SOLUBILITY • Effect of Temperature • Solids: generally increases with increasing temperature • • Note: not true for every solid (Figure 11. 6 pg 404) Gases: Less soluble in water as temperature increases • Ex. Carbonated drinks stay carbonated better when refrigerated • Effect of Pressure • Effects mainly the solubility of gases • Henry’s Law: Solubilty = k * P • k: solubility constant; P: partial pressure • If P is doubled, solubility doubles (tripled, solubility triples)

SOLUTIONS • Saturated solution: A solution containing the maxium possible amount of dissolved solute at equilibrium. • Supersaturated solution: A solution containing a greater than equilibrium amount of solute.

HENRY’S LAW EXAMPLE •

HENRY’S LAW EXAMPLE A) Air is a mixture of gases that is about 43. 0% N 2 by volume. When air is at standard pressure and 25. 0 ∘C , the N 2 component will dissolve in water with a solubility of 4. 88× 10 -4 M. What is the value of Henry's law constant for N 2 under these conditions? Solubility = k*P B) As a scuba diver descends under water, the pressure increases. At a total air pressure of 4. 65 atm and a temperature of 25. 0 ∘C , what is the solubility of N 2 in a diver's blood? (Use the k value determined from Part A).
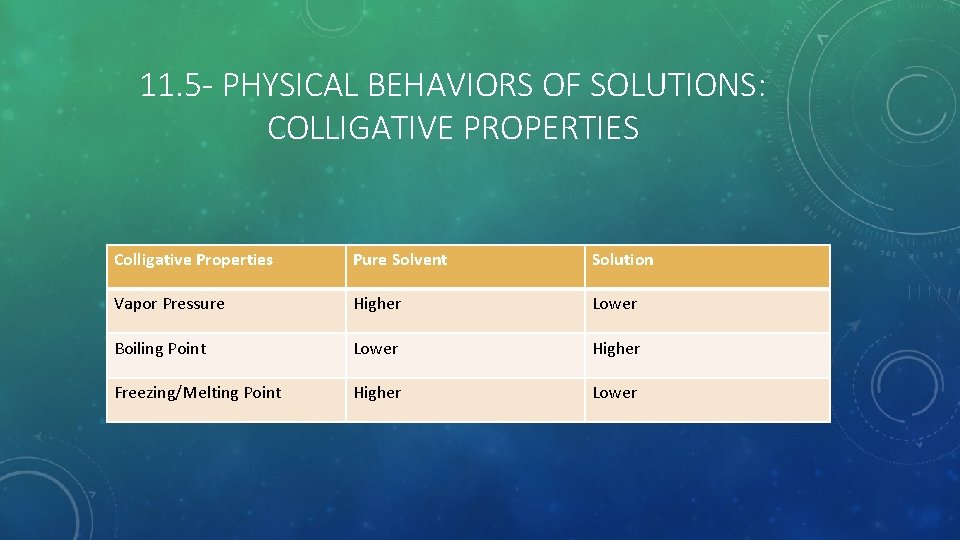
11. 5 - PHYSICAL BEHAVIORS OF SOLUTIONS: COLLIGATIVE PROPERTIES Colligative Properties Pure Solvent Solution Vapor Pressure Higher Lower Boiling Point Lower Higher Freezing/Melting Point Higher Lower

11. 6 - VAPOR-PRESSURE LOWERING OF SOLUTIONS: RAOULT’S LAW •

RAOULT’S LAW If 0. 880 mol of a nonvolatile nonelectrolyte are dissolved in 3. 80 mol of water, what is the vapor pressure PH 2 O of the resulting solution? The vapor pressure of pure water is 23. 8 torr at 25 ∘C
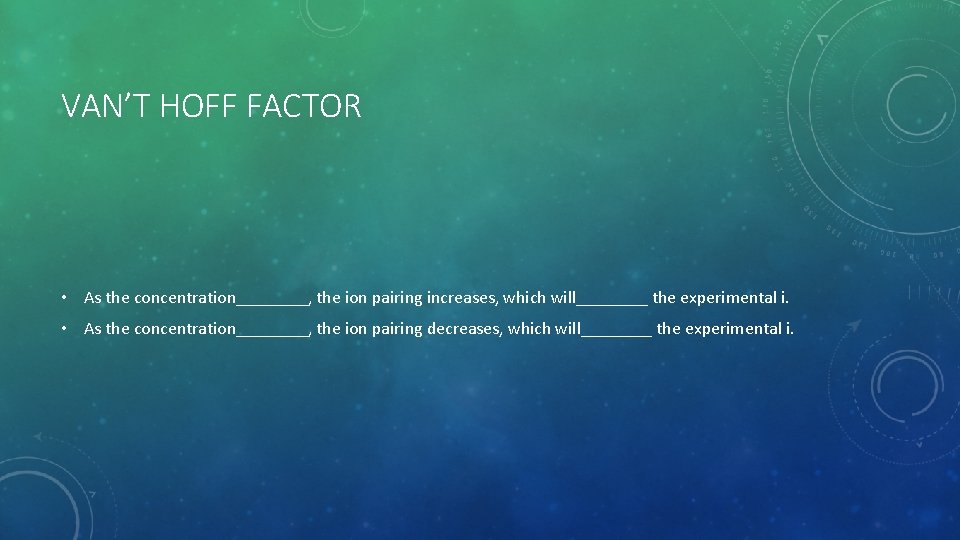
VAN’T HOFF FACTOR • As the concentration____, the ion pairing increases, which will____ the experimental i. • As the concentration____, the ion pairing decreases, which will____ the experimental i.

EXIT PROBLEM • 0. 78 g of a gas dissolves in 1. 0 L of water at 2. 2 atm, how much in g/L will dissolve if the pressure is raised to 7. 4 atm? Assume the temperature is held at a constant.
- Slides: 13