Chemistry 121122 Hydrogen Ions and Acidity Hydrogen Ions



![Acidic and Basic Solutions • A solution in which [H+] is greater than [OH−] Acidic and Basic Solutions • A solution in which [H+] is greater than [OH−]](https://slidetodoc.com/presentation_image/2f4cc1f68697169c7681745d55b55816/image-6.jpg)

![Calculating p. OH p. H + p. OH = 14 p. OH = -log[OH-] Calculating p. OH p. H + p. OH = 14 p. OH = -log[OH-]](https://slidetodoc.com/presentation_image/2f4cc1f68697169c7681745d55b55816/image-12.jpg)
![Calculating [H+] from p. H �Rearrange the equation p. H = -log[H+] = - Calculating [H+] from p. H �Rearrange the equation p. H = -log[H+] = -](https://slidetodoc.com/presentation_image/2f4cc1f68697169c7681745d55b55816/image-16.jpg)


- Slides: 23

Chemistry 121/122 Hydrogen Ions and Acidity

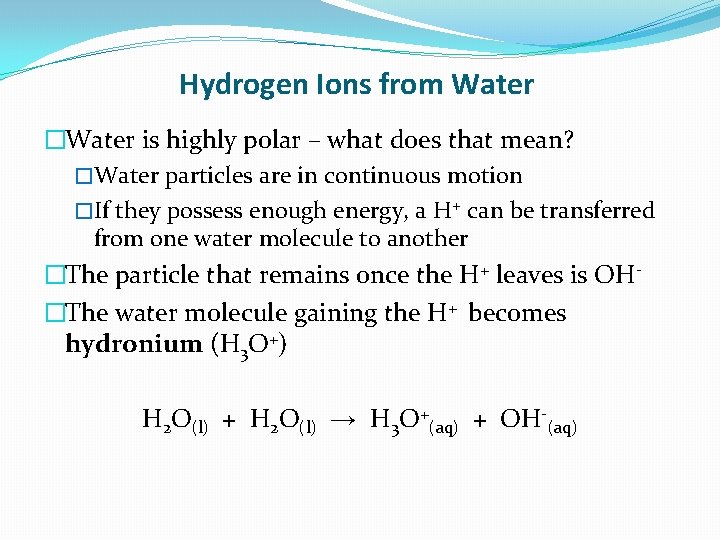
Hydrogen Ions from Water �Water is highly polar – what does that mean? �Water particles are in continuous motion �If they possess enough energy, a H+ can be transferred from one water molecule to another �The particle that remains once the H+ leaves is OH�The water molecule gaining the H+ becomes hydronium (H 3 O+) H 2 O(l) + H 2 O(l) → H 3 O+(aq) + OH-(aq)

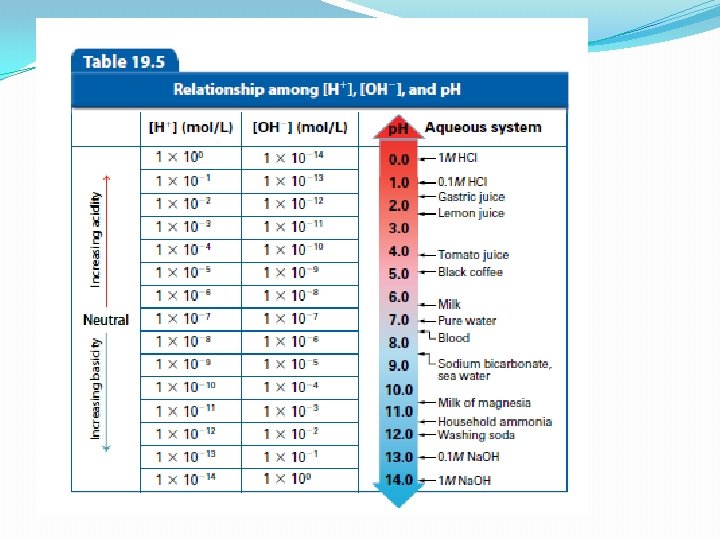
Self-ionization of water �Another way of writing the ionization of water is: H 2 O(l) ↔ H+(aq) + OH-(aq) �Hydronium and hydrogen ions can be written interchangeably �Both examples show water forming ions �The equilibrium concentration for both hydrogen and hydroxide ions is very small at 25°C �Each is only 1 x 10 -7 M �When both [H+] and [OH-] are equal in concentration, the solution is said to be neutral

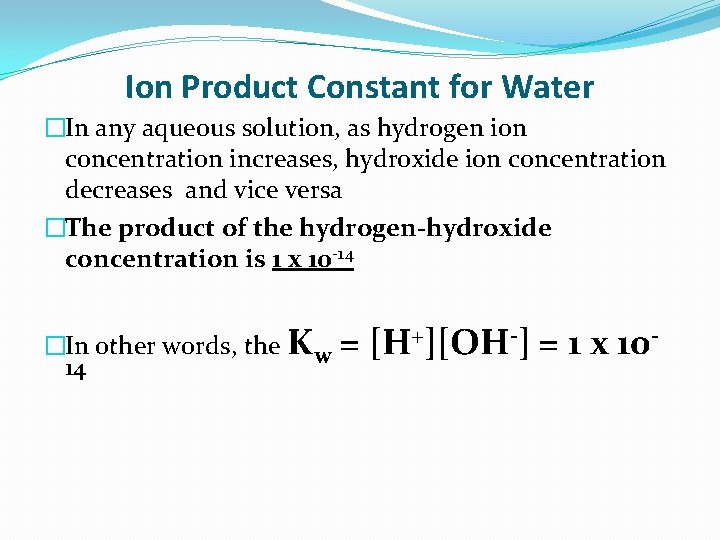
Ion Product Constant for Water �In any aqueous solution, as hydrogen ion concentration increases, hydroxide ion concentration decreases and vice versa �The product of the hydrogen-hydroxide concentration is 1 x 10 -14 �In other words, the Kw 14 = [H+][OH-] = 1 x 10 -

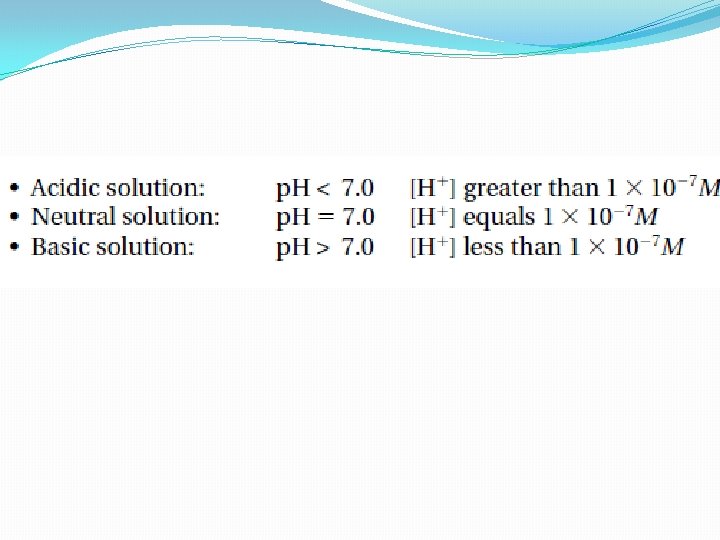
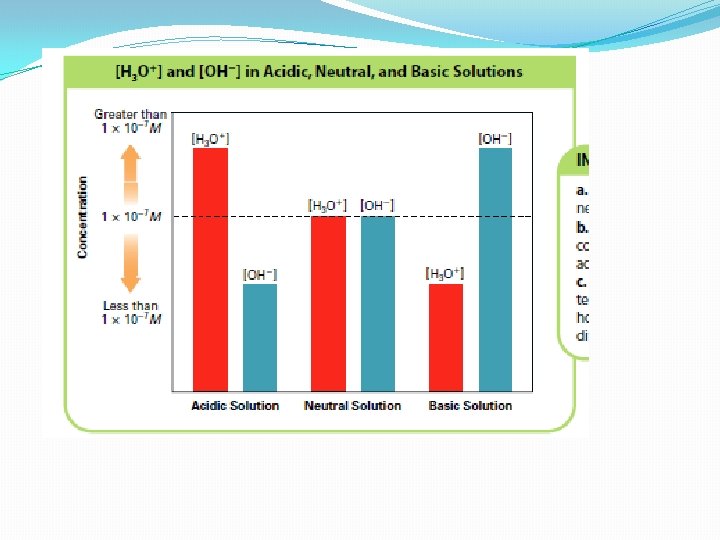
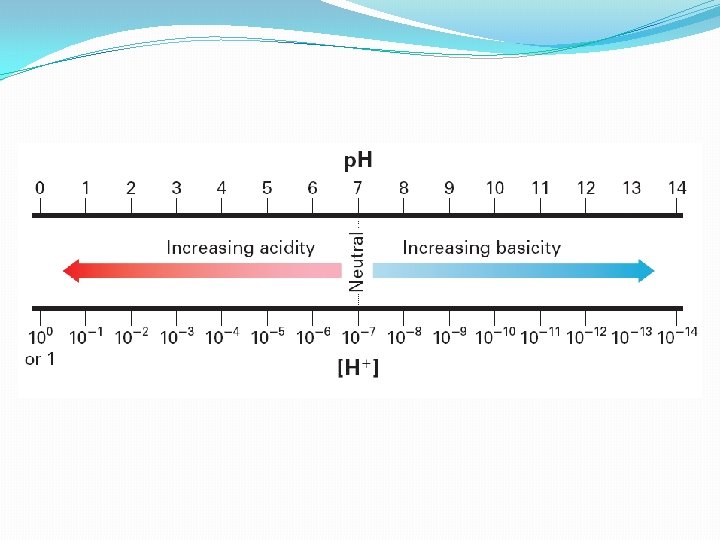
Acidic and Basic Solutions �When acids form aqueous solutions, there is more H+ ions than OH- (coming from the ionization of water) �The [H+] is greater than 1 x 10 -7 M �Basic solutions are the opposite �The hydroxide ion concentration is greater than the hydrogen ion concentration �The [OH-] is greater than 1 x 10 -7 M ( The [H+] is less than 1 × 10− 7 M) �They are otherwise known as alkaline solutions
![Acidic and Basic Solutions A solution in which H is greater than OH Acidic and Basic Solutions • A solution in which [H+] is greater than [OH−]](https://slidetodoc.com/presentation_image/2f4cc1f68697169c7681745d55b55816/image-6.jpg)
Acidic and Basic Solutions • A solution in which [H+] is greater than [OH−] is an acidic solution. The [H+] is greater than 1 × 10− 7 M. • A basic solution is one in which [H+] is less than [OH−]. The [H+] is less than 1 × 10− 7 M –Basic solutions are also known as alkaline solutions


Sample Problem, p. 596 Complete 9, 10, p. 596

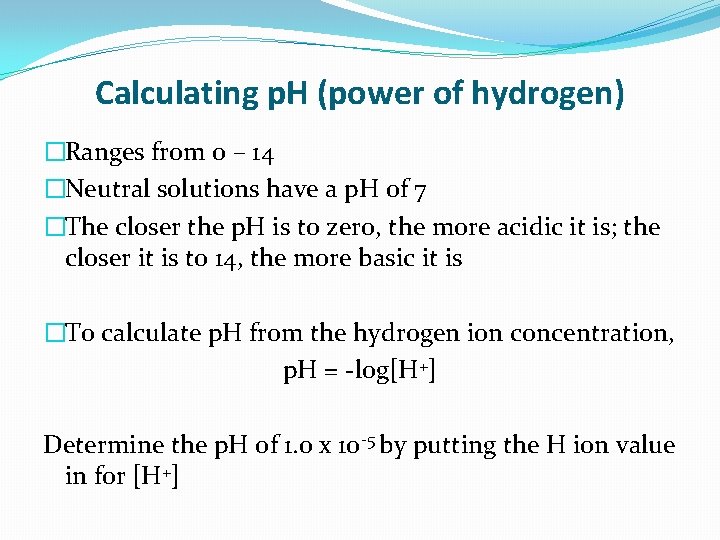
Calculating p. H (power of hydrogen) �Ranges from 0 – 14 �Neutral solutions have a p. H of 7 �The closer the p. H is to zero, the more acidic it is; the closer it is to 14, the more basic it is �To calculate p. H from the hydrogen ion concentration, p. H = -log[H+] Determine the p. H of 1. 0 x 10 -5 by putting the H ion value in for [H+]


![Calculating p OH p H p OH 14 p OH logOH Calculating p. OH p. H + p. OH = 14 p. OH = -log[OH-]](https://slidetodoc.com/presentation_image/2f4cc1f68697169c7681745d55b55816/image-12.jpg)
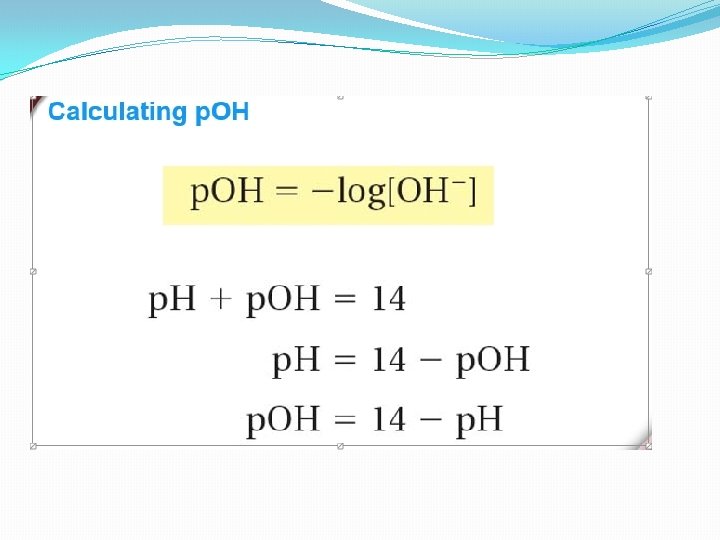
Calculating p. OH p. H + p. OH = 14 p. OH = -log[OH-] �Express concentrations in scientific notation �When writing the p. H or p. OH, the concentration of the solution is equivalent to the same number of digits after the decimal of the value itself �Ex. 1. 0 x 10 -5 = 5. 00 (two digits in the base of the scientific notation – two digits after the decimal)



When is p. H not a whole number? �Most of the time… As a result, it is not easy to make a simple mental calculation. Instead, use the logarithmic equation to convert from concentration to p. H or p. OH. Ex. [H+] p. H = 4. 2 x 10 -10 M = -log[4. 2 x 10 -10] = 9. 37675 = 9. 38
![Calculating H from p H Rearrange the equation p H logH Calculating [H+] from p. H �Rearrange the equation p. H = -log[H+] = -](https://slidetodoc.com/presentation_image/2f4cc1f68697169c7681745d55b55816/image-16.jpg)
Calculating [H+] from p. H �Rearrange the equation p. H = -log[H+] = - antilog(10 X) p. H Using Kw, in addition to p. H/p. OH, [H+]/[OH-], you can solve for any unknown Ex. p. H = 6. 35 [H+] = 10 x -6. 35 =4. 5 x 10 -7 M

Assignment 9 -10, p 596 11 -12, p. 599 13 -14 p 600 15 -16 p 601 Worksheet


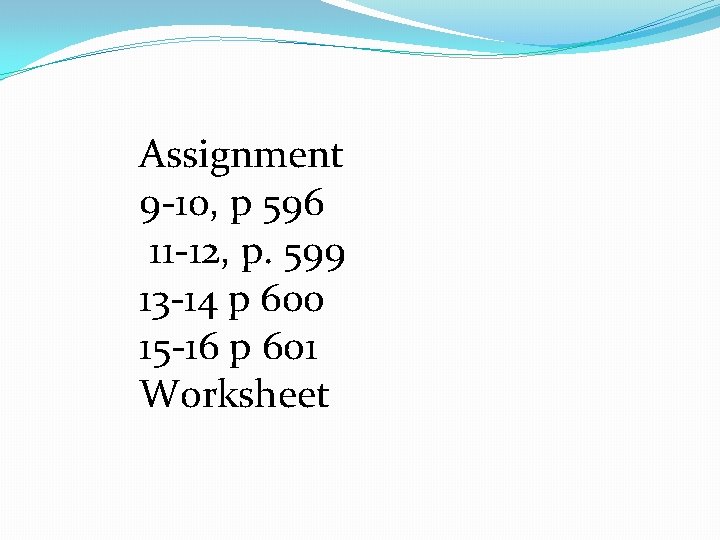
Sample Problem 19. 3, p. 600

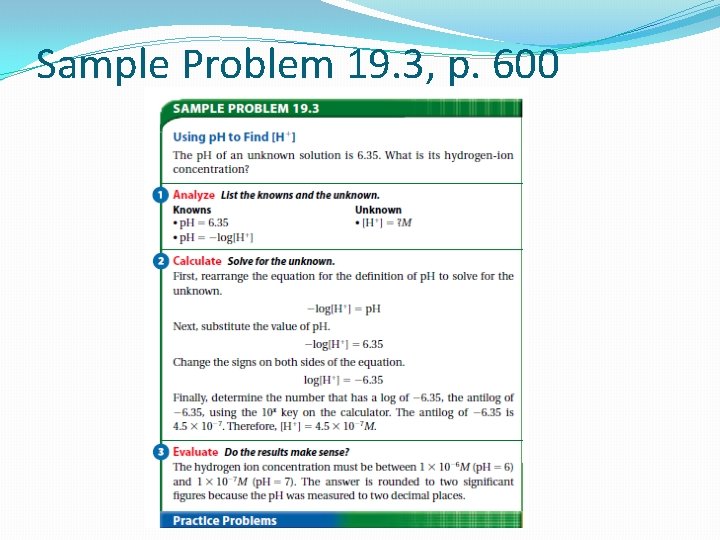
Sample Problem 19. 4, p. 601 Questions 15 -16, p. 601

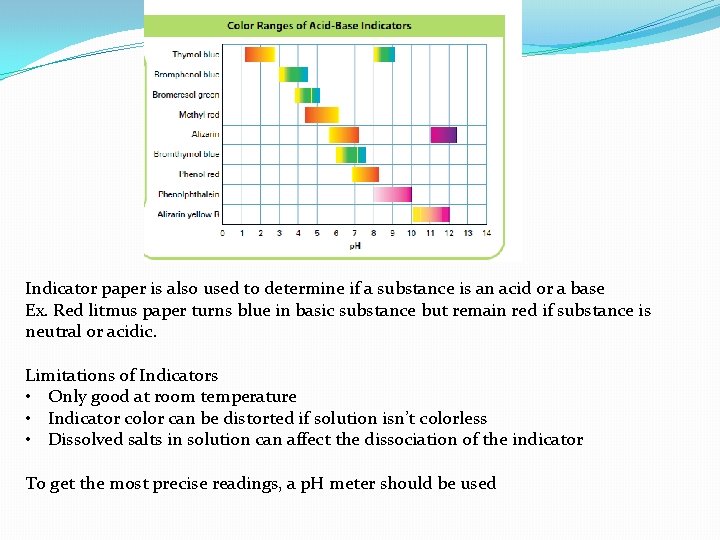
Acid-Base Indicators �An indicator (HIn) is an acid or base that undergoes dissociation in a known p. H range �They are valuable tools because its acid form and base form have different colors in solution �Most indicators have a narrow p. H range that they detect (2 p. H units) examples �Bromophenopl blue aprrox. 2. 9 -4. 5 �Phenolphthalein is colourless up to p. H 8 and eventually turns bright pink at p. H 10

Indicator paper is also used to determine if a substance is an acid or a base Ex. Red litmus paper turns blue in basic substance but remain red if substance is neutral or acidic. Limitations of Indicators • Only good at room temperature • Indicator color can be distorted if solution isn’t colorless • Dissolved salts in solution can affect the dissociation of the indicator To get the most precise readings, a p. H meter should be used

Assignment Page 601 #15 & 16 Page 604 #17 - 21 Practice Problems 19. 1 & 19. 2