Chemistry 120 Scaling a graph Outline I Scaling
- Slides: 8

Chemistry 120 Scaling a graph Outline I. Scaling a graph II. Determination of equation of line

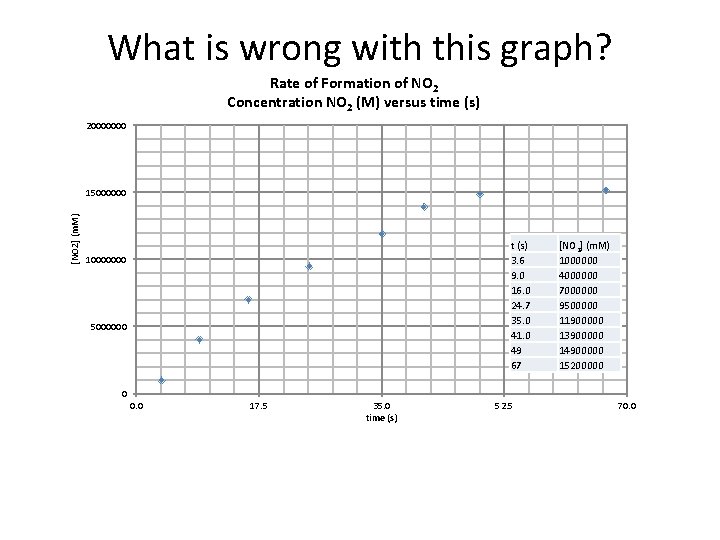
What is wrong with this graph? Rate of Formation of NO 2 Concentration NO 2 (M) versus time (s) 20000000 [NO 2] (m. M) 15000000 t (s) 3. 6 9. 0 16. 0 24. 7 35. 0 41. 0 49 67 10000000 5000000 [NO 2] (m. M) 1000000 4000000 7000000 9500000 11900000 13900000 14900000 15200000 0 0. 0 17. 5 35. 0 time (s) 52. 5 70. 0

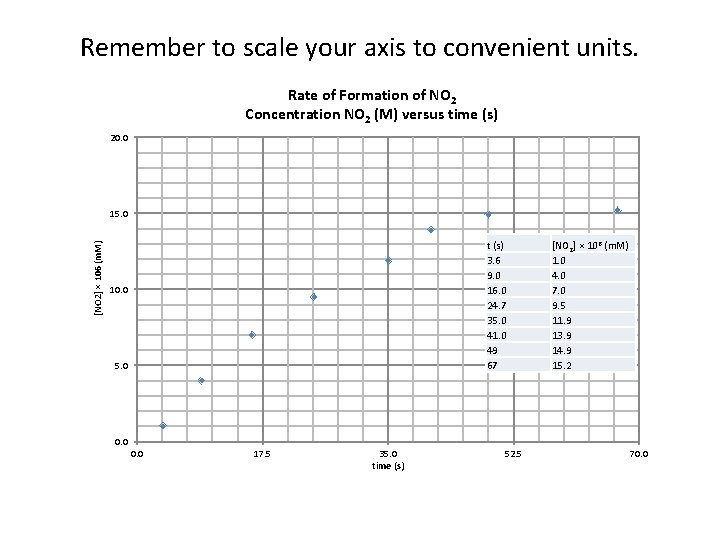
Remember to scale your axis to convenient units. Rate of Formation of NO 2 Concentration NO 2 (M) versus time (s) 20. 0 [NO 2] × 106 (m. M) 15. 0 t (s) 3. 6 9. 0 16. 0 24. 7 35. 0 41. 0 49 67 10. 0 5. 0 [NO 2] × 106 (m. M) 1. 0 4. 0 7. 0 9. 5 11. 9 13. 9 14. 9 15. 2 0. 0 17. 5 35. 0 time (s) 52. 5 70. 0

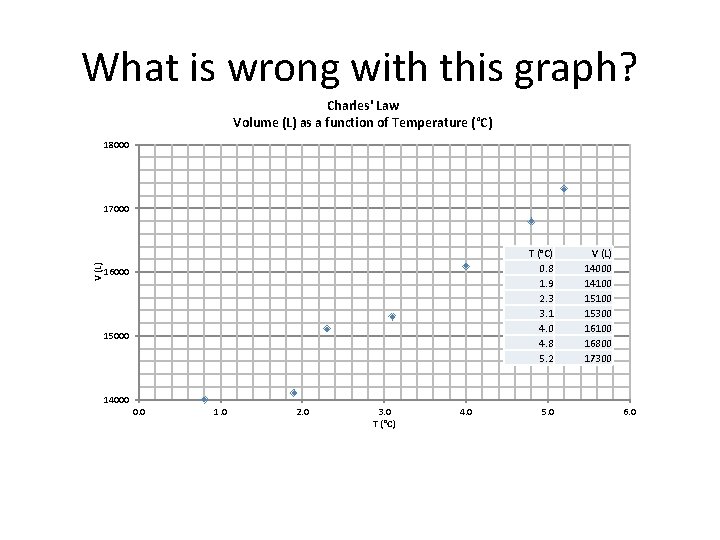
What is wrong with this graph? Charles' Law Volume (L) as a function of Temperature (°C) 18000 V (L) 17000 T (°C) 0. 8 1. 9 2. 3 3. 1 4. 0 4. 8 5. 2 16000 15000 V (L) 14000 14100 15300 16100 16800 17300 14000 0. 0 1. 0 2. 0 3. 0 T (°C) 4. 0 5. 0 6. 0 R 2 = 0. 956

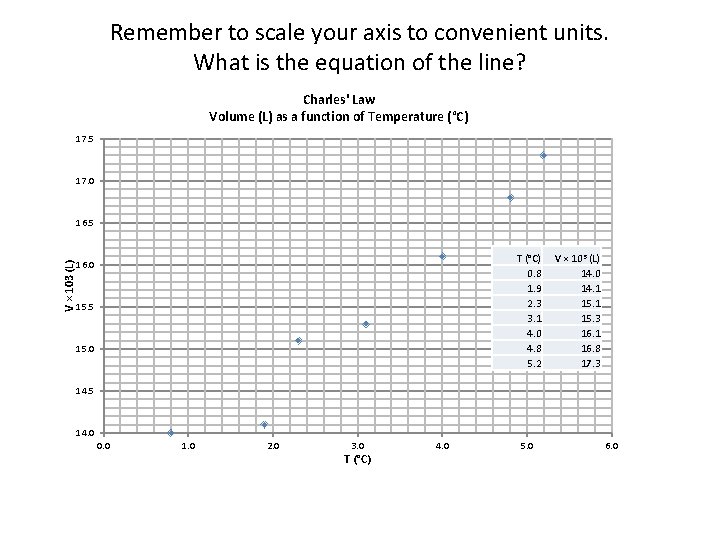
Remember to scale your axis to convenient units. What is the equation of the line? Charles' Law Volume (L) as a function of Temperature (°C) 17. 5 17. 0 V × 103 (L) 16. 5 T (°C) 0. 8 1. 9 2. 3 3. 1 4. 0 4. 8 5. 2 16. 0 15. 5 15. 0 V × 103 (L) 14. 0 14. 1 15. 3 16. 1 16. 8 17. 3 14. 5 14. 0 0. 0 1. 0 2. 0 3. 0 T (°C) 4. 0 5. 0 6. 0

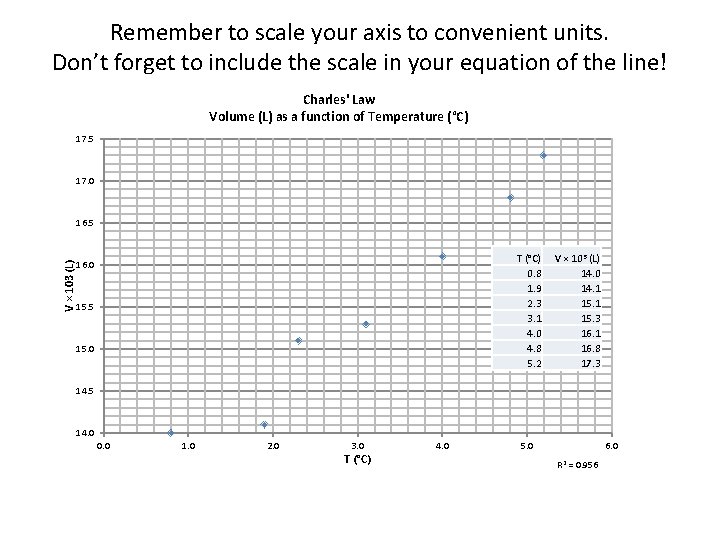
Remember to scale your axis to convenient units. Don’t forget to include the scale in your equation of the line! Charles' Law Volume (L) as a function of Temperature (°C) 17. 5 17. 0 V × 103 (L) 16. 5 T (°C) 0. 8 1. 9 2. 3 3. 1 4. 0 4. 8 5. 2 16. 0 15. 5 15. 0 V × 103 (L) 14. 0 14. 1 15. 3 16. 1 16. 8 17. 3 14. 5 14. 0 0. 0 1. 0 2. 0 3. 0 T (°C) 4. 0 5. 0 6. 0 R 2 = 0. 956

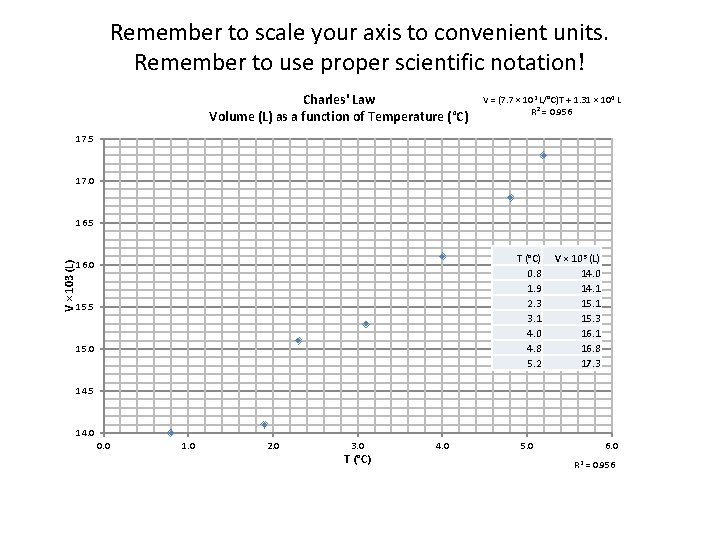
Remember to scale your axis to convenient units. Remember to use proper scientific notation! Charles' Law Volume (L) as a function of Temperature (°C) V = (7. 7 × 102 L/°C)T + 1. 31 × 104 L R² = 0. 956 17. 5 17. 0 V × 103 (L) 16. 5 T (°C) 0. 8 1. 9 2. 3 3. 1 4. 0 4. 8 5. 2 16. 0 15. 5 15. 0 V × 103 (L) 14. 0 14. 1 15. 3 16. 1 16. 8 17. 3 14. 5 14. 0 0. 0 1. 0 2. 0 3. 0 T (°C) 4. 0 5. 0 6. 0 R 2 = 0. 956

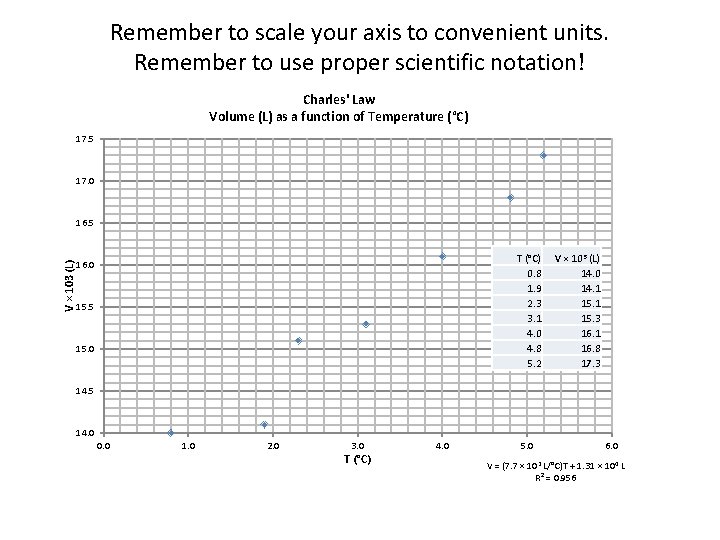
Remember to scale your axis to convenient units. Remember to use proper scientific notation! Charles' Law Volume (L) as a function of Temperature (°C) 17. 5 17. 0 V × 103 (L) 16. 5 T (°C) 0. 8 1. 9 2. 3 3. 1 4. 0 4. 8 5. 2 16. 0 15. 5 15. 0 V × 103 (L) 14. 0 14. 1 15. 3 16. 1 16. 8 17. 3 14. 5 14. 0 0. 0 1. 0 2. 0 3. 0 T (°C) 4. 0 5. 0 6. 0 V = (7. 7 × 102 L/°C)T + 1. 31 × 104 L R² = 0. 956