Chemistry 120 Chapter 6 Chemical Nomenclature Outline I













- Slides: 50

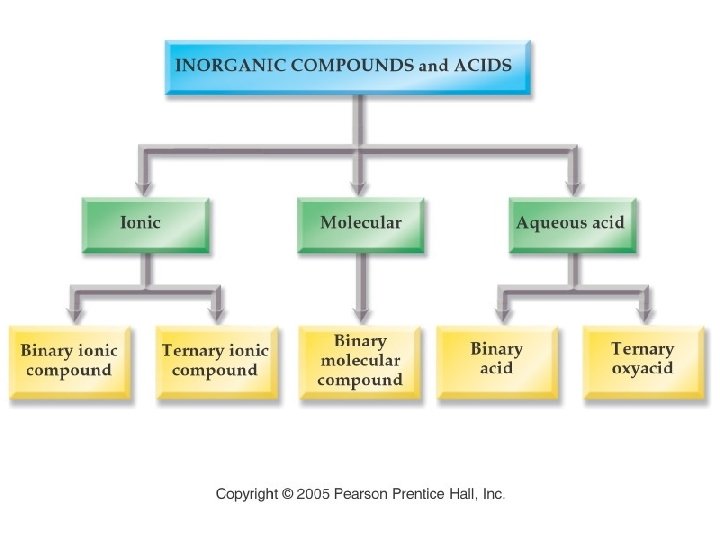
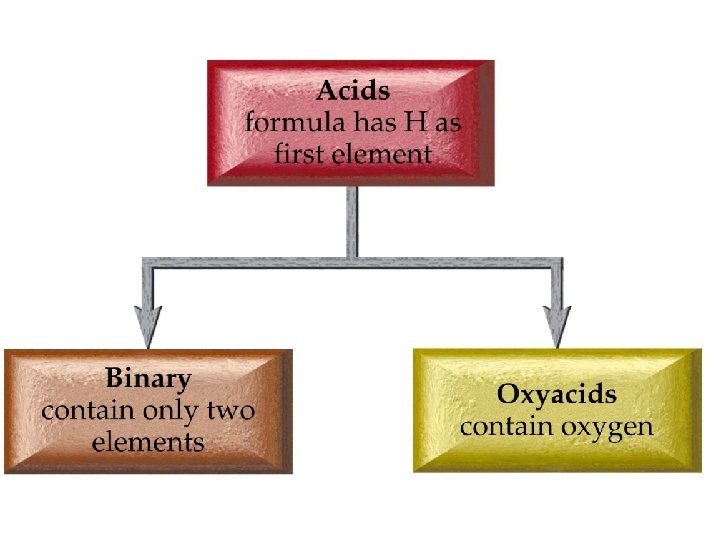
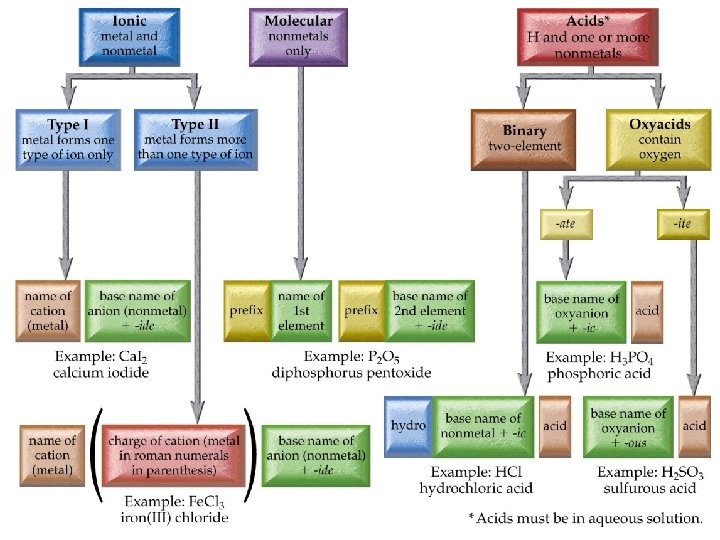
Chemistry 120 Chapter 6: Chemical Nomenclature Outline I. Octet Rule II. Ionic Compounds A. Binary Nomenclature B. Polyatomic Nomenclature III. Covalent Compounds A. Binary Nomenclature IV. Acids A. Binary Nomenclature B. Ternary Nomenclature (Oxyacids) V. Hydrates

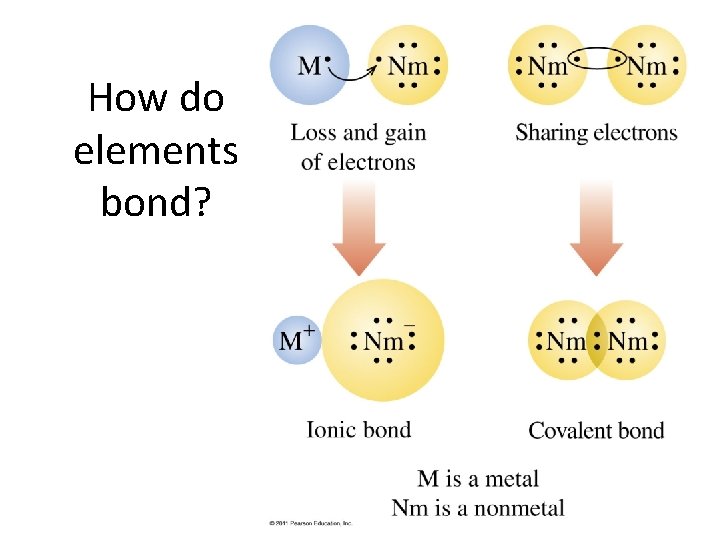
How do elements bond?


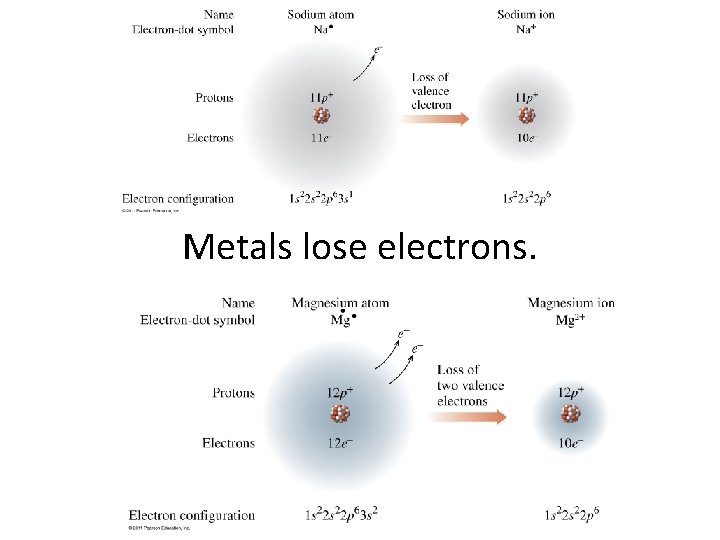
Metals lose electrons.

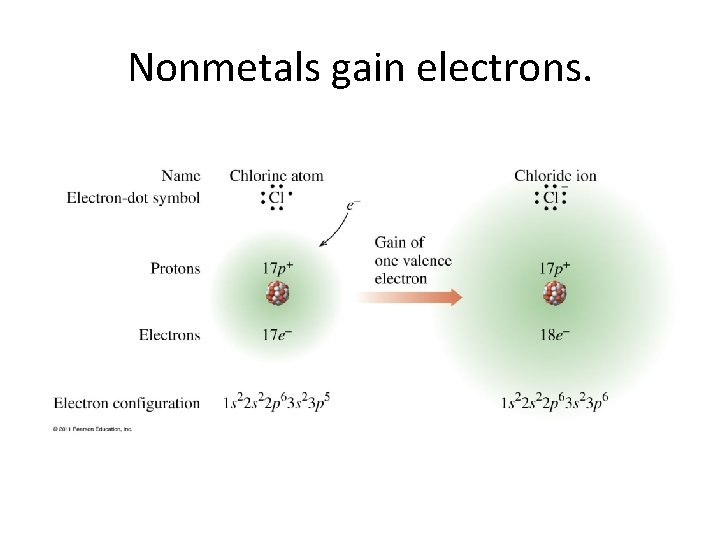
Nonmetals gain electrons.

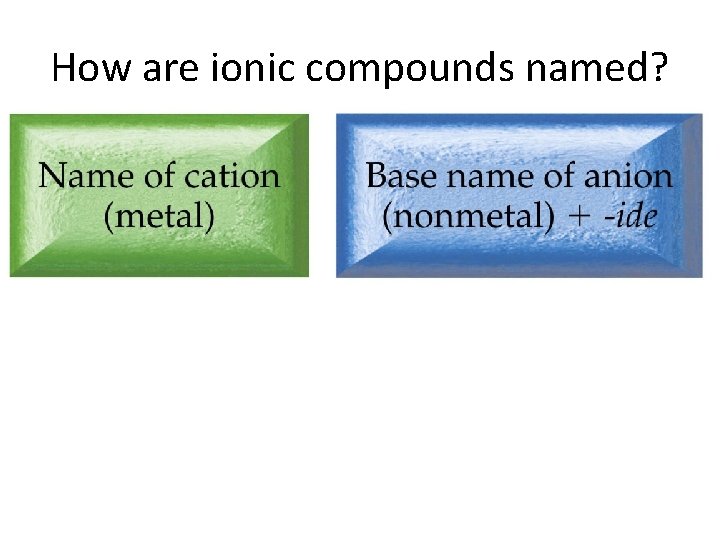
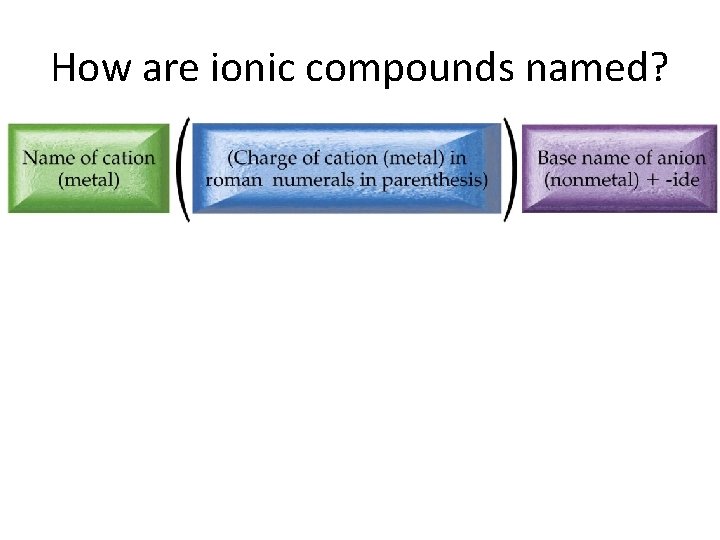
How are ionic compounds named?


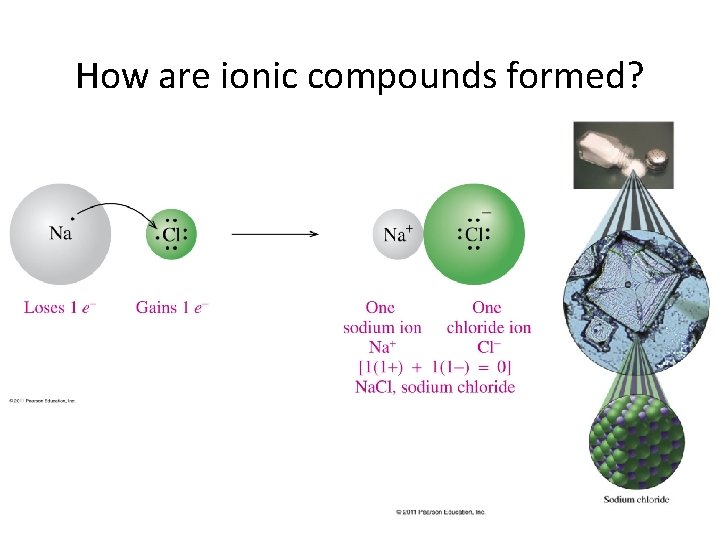
How are ionic compounds formed?

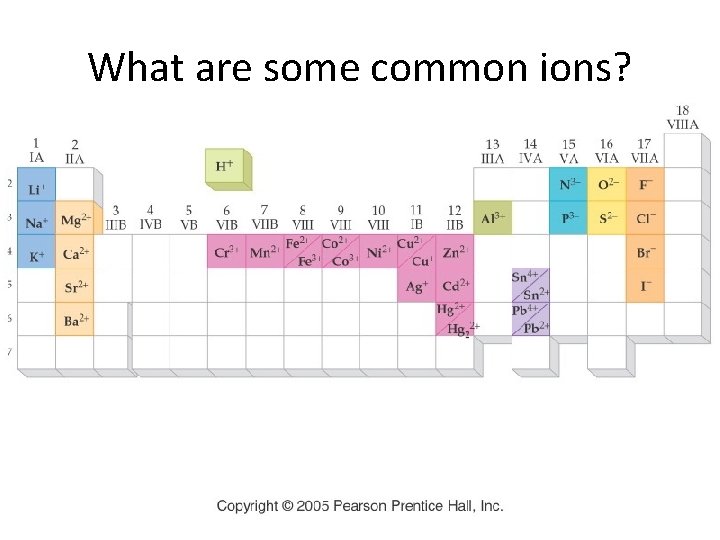
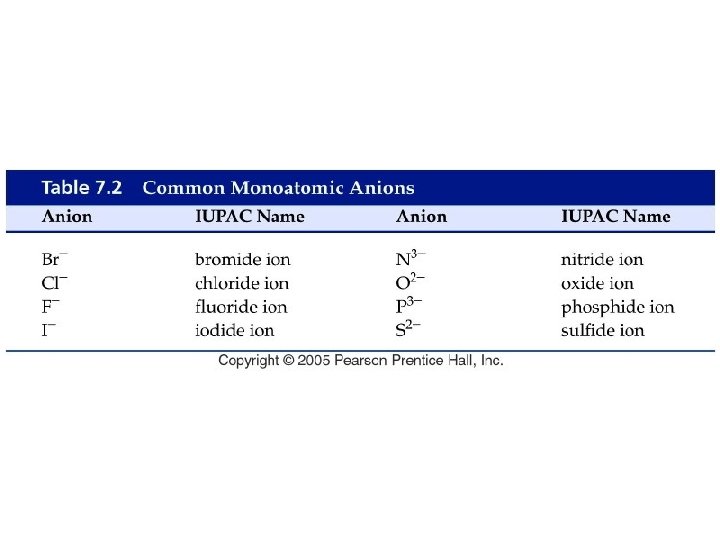
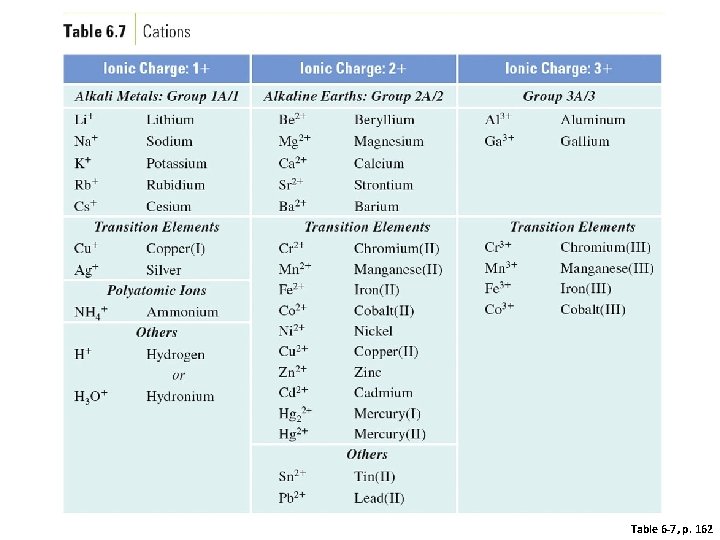
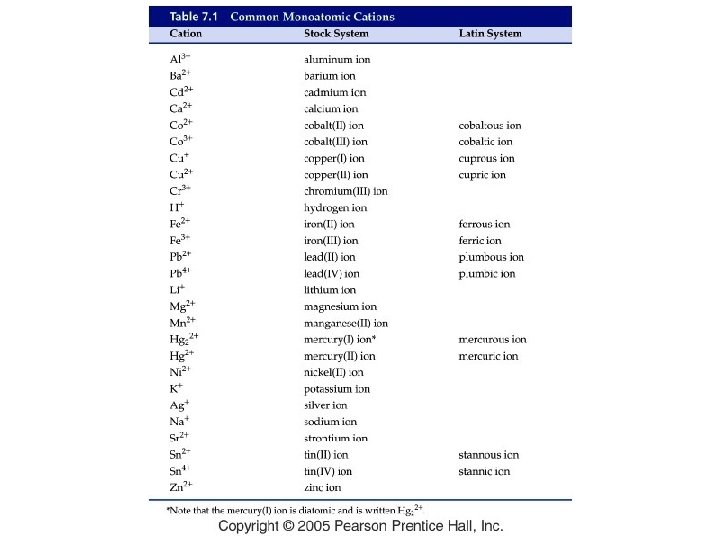
What are some common ions?


Table 6 -7, p. 162


How are ionic compounds named?

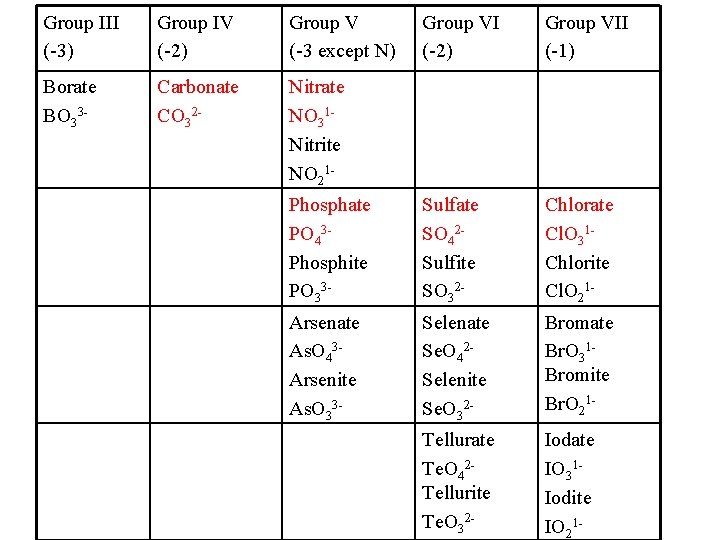
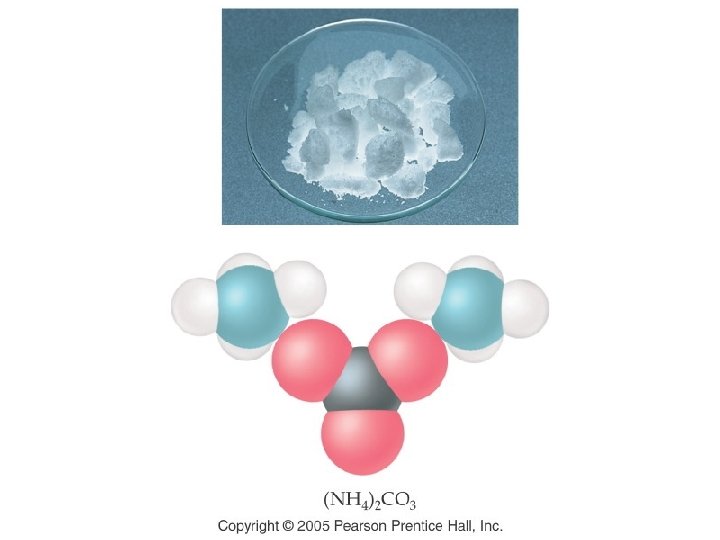
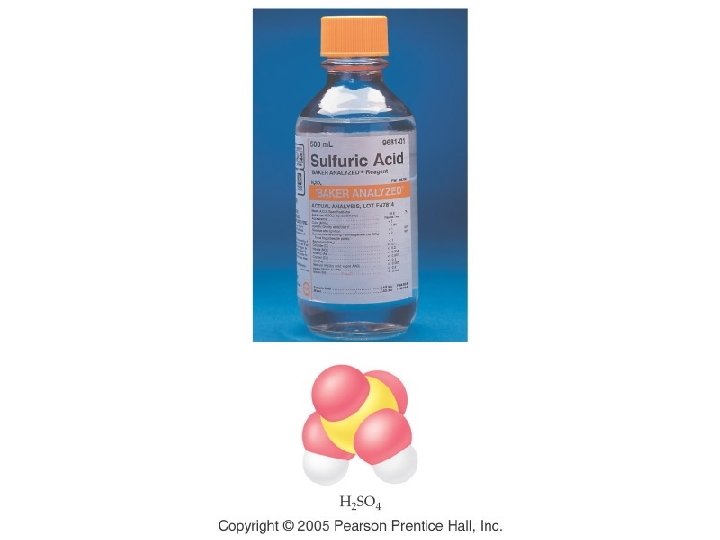
Group III (-3) Group IV (-2) Group V (-3 except N) Group VI (-2) Group VII (-1) Borate BO 33 - Carbonate CO 32 - Nitrate NO 31 Nitrite NO 21 Phosphate PO 43 Phosphite PO 33 - Sulfate SO 42 Sulfite SO 32 - Chlorate Cl. O 31 Chlorite Cl. O 21 - Arsenate As. O 43 Arsenite As. O 33 - Selenate Se. O 42 Selenite Se. O 32 - Bromate Br. O 31 Bromite Br. O 21 - Tellurate Te. O 42 Tellurite Te. O 32 - Iodate IO 31 Iodite IO 21 -

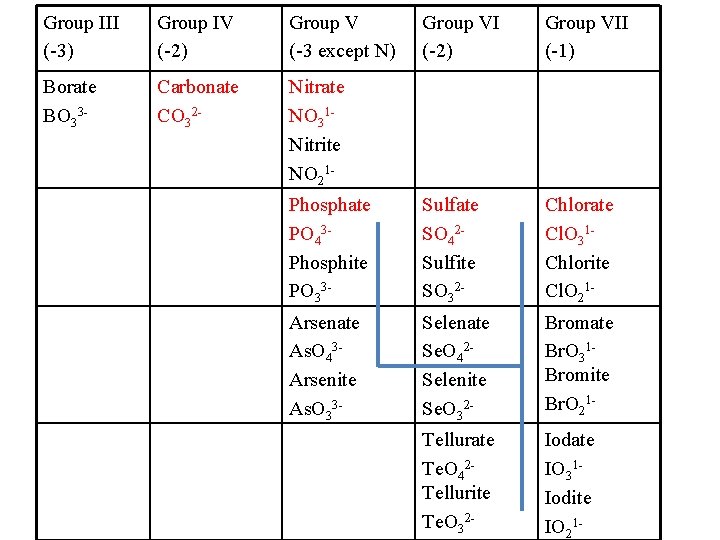
Group III (-3) Group IV (-2) Group V (-3 except N) Group VI (-2) Group VII (-1) Borate BO 33 - Carbonate CO 32 - Nitrate NO 31 Nitrite NO 21 Phosphate PO 43 Phosphite PO 33 - Sulfate SO 42 Sulfite SO 32 - Chlorate Cl. O 31 Chlorite Cl. O 21 - Arsenate As. O 43 Arsenite As. O 33 - Selenate Se. O 42 Selenite Se. O 32 - Bromate Br. O 31 Bromite Br. O 21 - Tellurate Te. O 42 Tellurite Te. O 32 - Iodate IO 31 Iodite IO 21 -

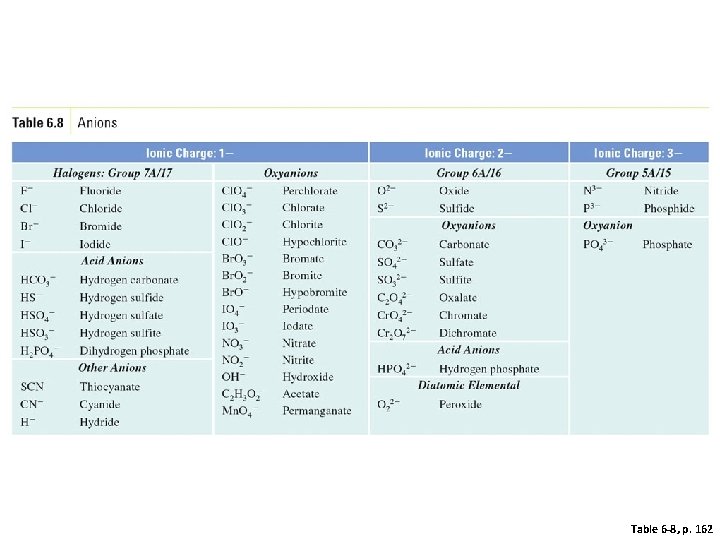
Table 6 -8, p. 162


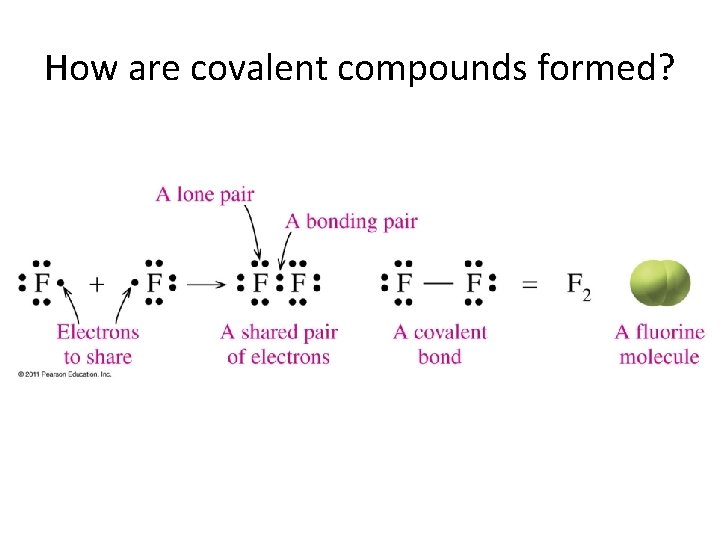
How are covalent compounds formed?

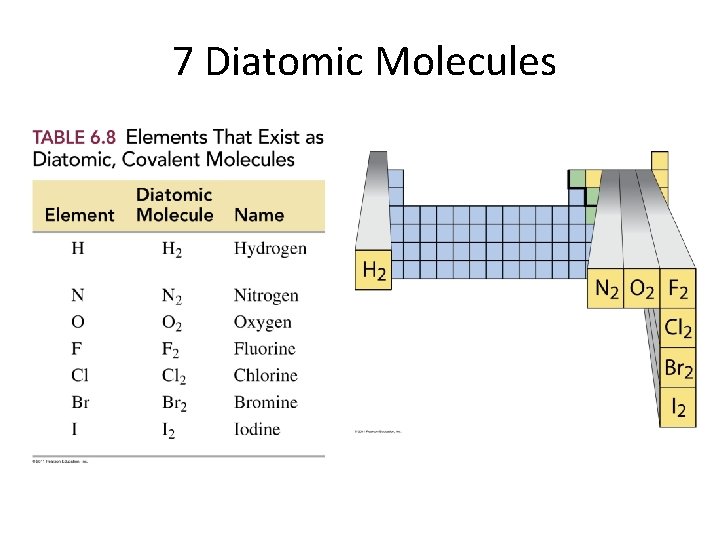
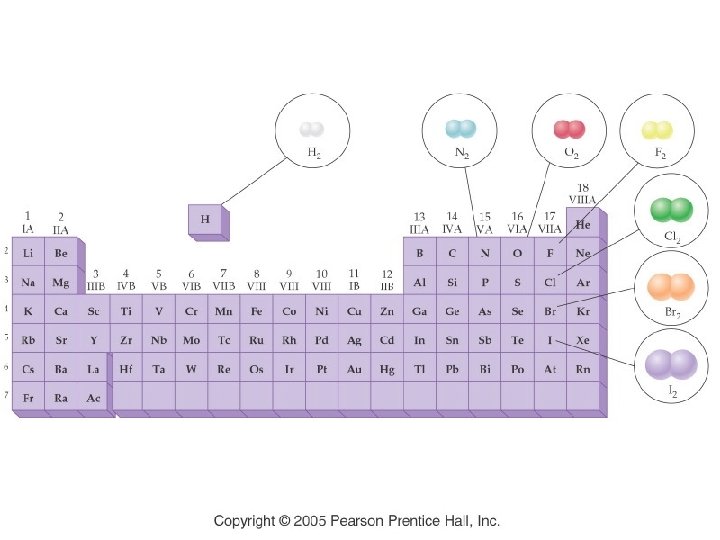
7 Diatomic Molecules


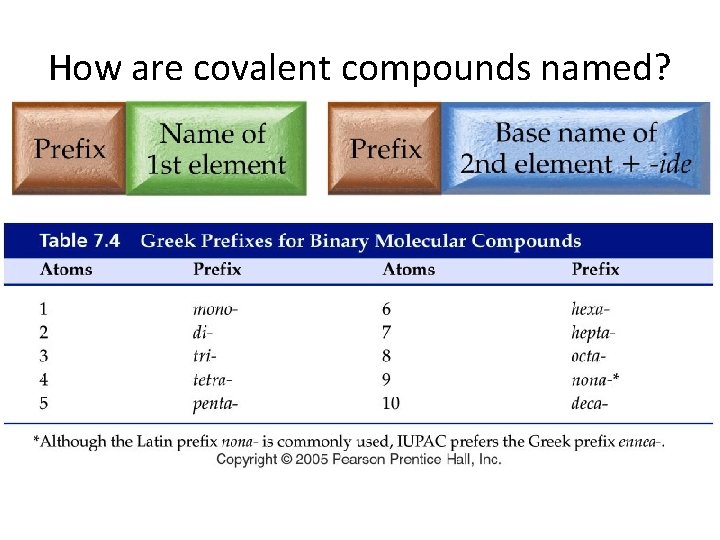
How are covalent compounds named?


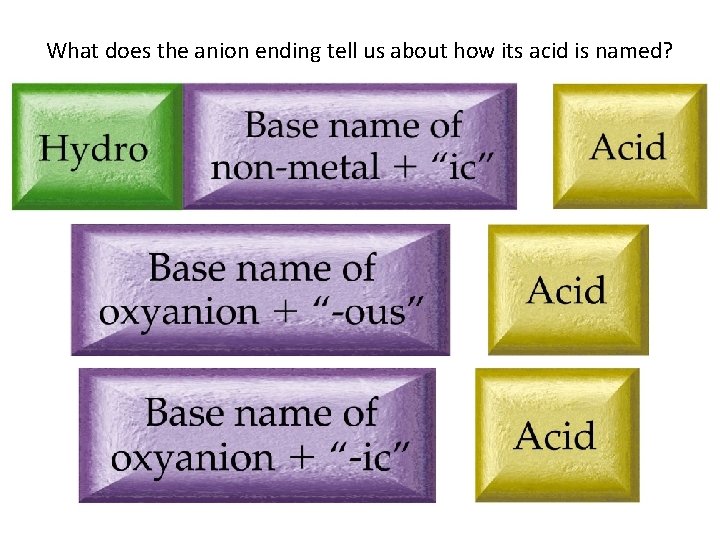
What does the anion ending tell us about how its acid is named?

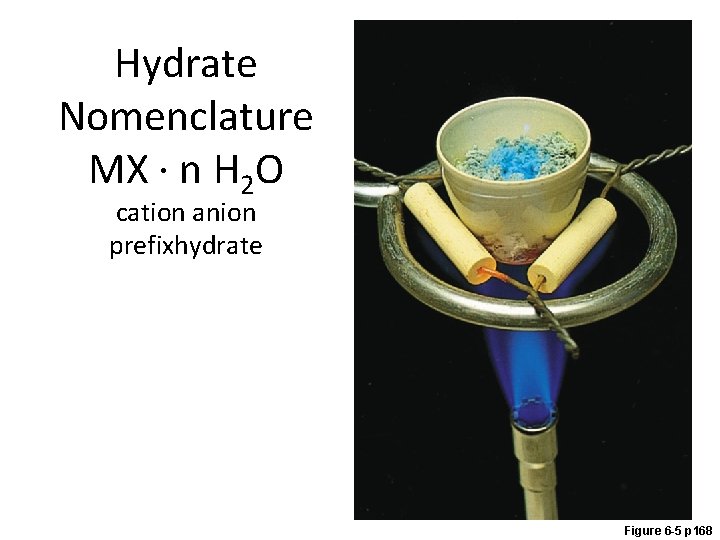
Hydrate Nomenclature MX · n H 2 O cation anion prefixhydrate Figure 6 -5 p 168




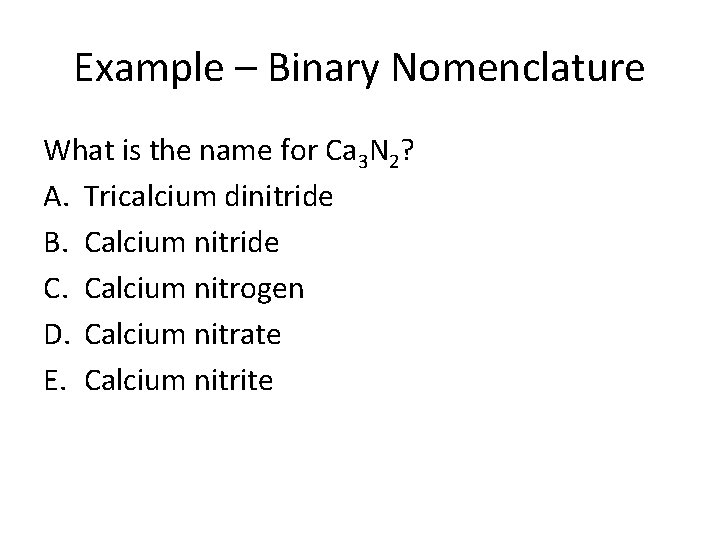
Example – Binary Nomenclature What is the name for Ca 3 N 2? A. Tricalcium dinitride B. Calcium nitride C. Calcium nitrogen D. Calcium nitrate E. Calcium nitrite

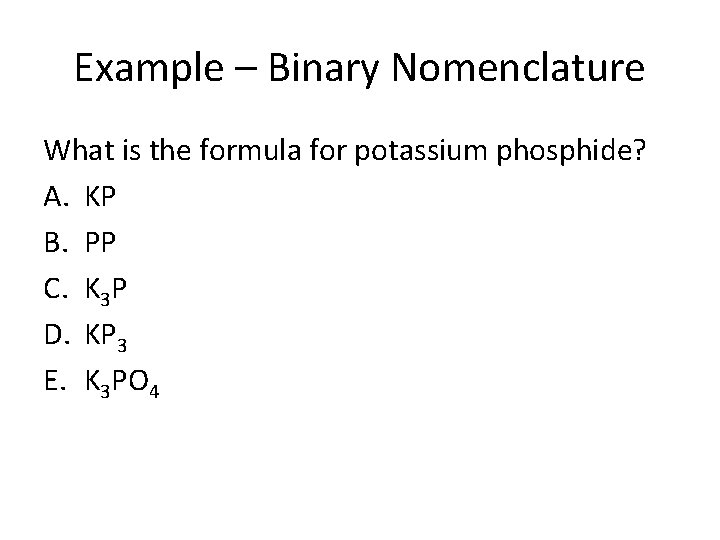
Example – Binary Nomenclature What is the formula for potassium phosphide? A. KP B. PP C. K 3 P D. KP 3 E. K 3 PO 4


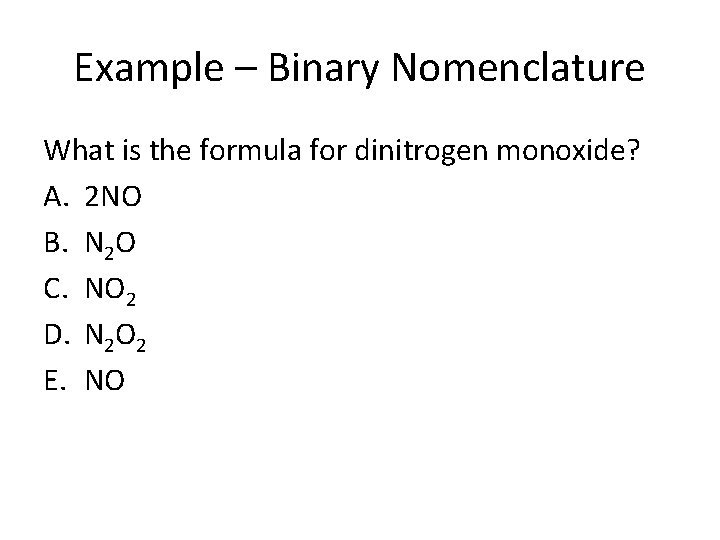
Example – Binary Nomenclature What is the formula for dinitrogen monoxide? A. 2 NO B. N 2 O C. NO 2 D. N 2 O 2 E. NO

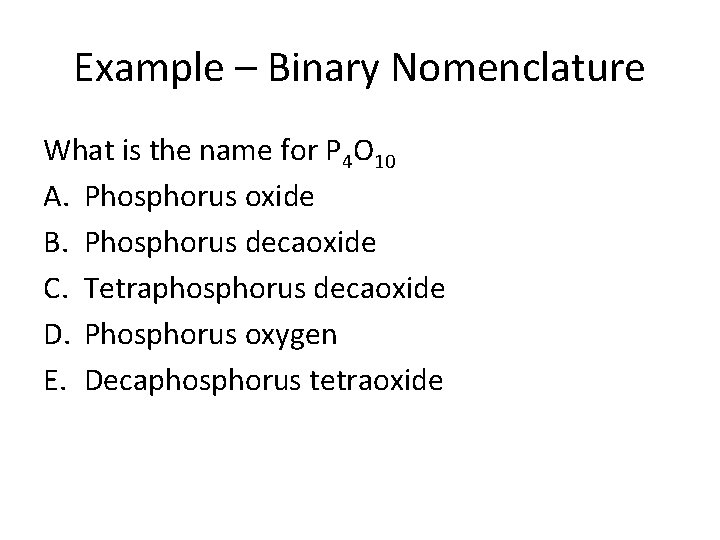
Example – Binary Nomenclature What is the name for P 4 O 10 A. Phosphorus oxide B. Phosphorus decaoxide C. Tetraphosphorus decaoxide D. Phosphorus oxygen E. Decaphosphorus tetraoxide




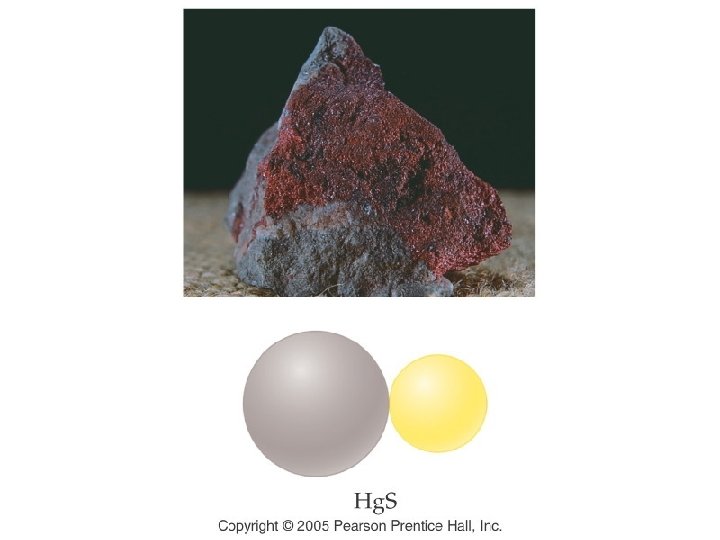
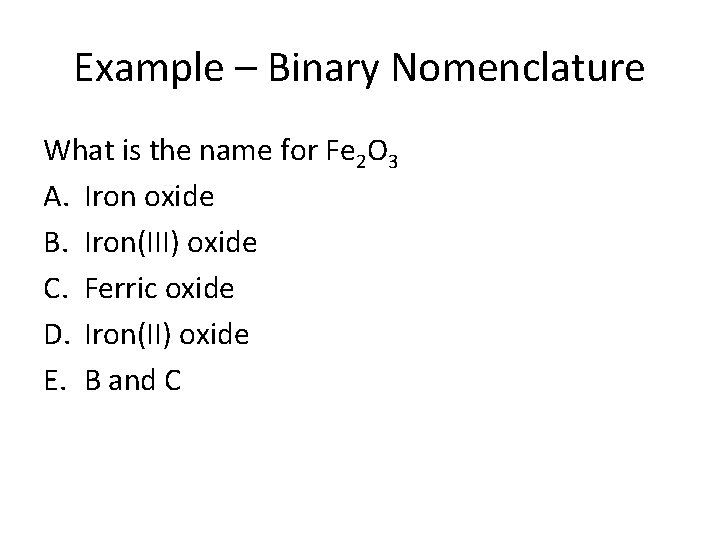
Example – Binary Nomenclature What is the name for Fe 2 O 3 A. Iron oxide B. Iron(III) oxide C. Ferric oxide D. Iron(II) oxide E. B and C

Example – Binary Nomenclature What is the formula for lead(IV) oxide? A. Pb 2 O 4 B. Pb. O 2 C. Pb. O D. Lb. O 2 E. Plumbic oxide




Example – Ternary and Quaternary Nomenclature What is the name of Na. NO 3? A. Sodium nitrite B. Sodium nitride C. Sodium nitrogen trioxide D. Sodium nitrate E. Sodium nitrogen oxide

Example – Ternary and Quaternary Nomenclature What is the name of Cr 2(CO 3)3? A. Chromium carbonate B. Chromium(II) carbonate C. Chromium(II) carbonate(III) D. Chromium(III) carbon trioxide E. Chromium(III) carbonate



Example – Acid Nomenclature What is the name of HCl (aq)? A. Chloric acid B. Hydrochloric acid C. Hydrogen chloride D. Hydrochlorous acid E. B and C

Example – Acid Nomenclature What is the name of HCl. O 3 (aq)? A. Chloric acid B. Hydrochloric acid C. Hydrogen chlorate D. chlorous acid E. Perchloric acid

Example – Acid Nomenclature What is the formula for phosphorous acid? A. H 3 PO 3 (aq) B. H 3 P (aq) C. H 3 PO 4 (aq) D. PO 3 (aq) E. H 2 PO 3 (aq)

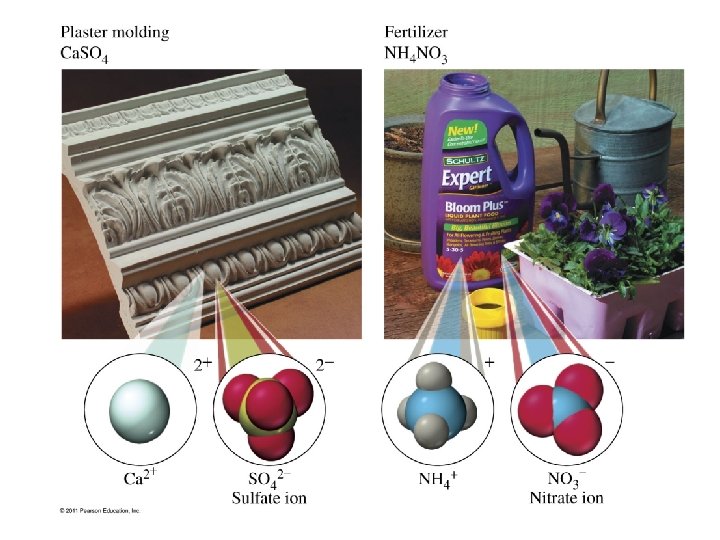
What is the systematic name of gypsum, Ca. SO 4 · 2 H 2 O? p 169

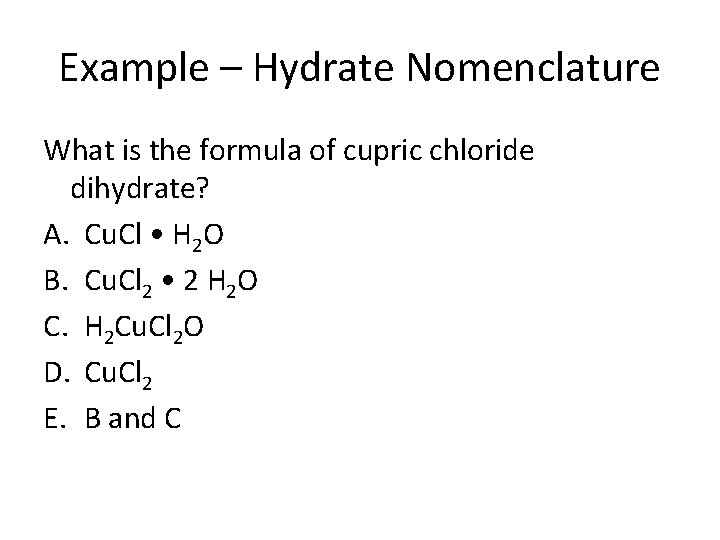
Example – Hydrate Nomenclature What is the formula of cupric chloride dihydrate? A. Cu. Cl • H 2 O B. Cu. Cl 2 • 2 H 2 O C. H 2 Cu. Cl 2 O D. Cu. Cl 2 E. B and C

Example – Hydrate Nomenclature What is the name of Mg. SO 4 • 6 H 2 O? A. Magnesium sulfate hexahydrate B. Manganese(II) sulfate hexahydrate C. Magnesium sulfate hydrate D. Magnesium(II) sulfate hexahydrate E. Magnesium(II) sulfite hexahydrate