Chemistry 12 1 Stoichiometry The arithmetic of equations








- Slides: 12

Chemistry 12. 1 Stoichiometry “The arithmetic of equations”

Chapter 12 Objective This unit will allow you to calculate the amounts of chemical substances involved in a chemical reaction. You will use balanced chemical equations to do this…

Tiny Tricycles – 12. 1 wks… Cookie Example

B. Cookie Example 1. Recipe… 2 large eggs + 1/2 cup sugar + 1 teaspoon vanilla + 3 tbsp cocoa powder + 1/2 cup chocolate chips 8 chocolate chip cookies 2. You have… 16 large eggs 12 cups sugar 6 teaspoons vanilla 33 tbsp cocoa powder 18 cups chocolate chips ? chocolate chip cookies 48 chocolate chip cookies

3. What do you have left? 4 eggs 9 cups of sugar 15 tbsp of cocoa powder 15 cups of chocolate chips 4. What do you need for 4 dozen cookies? 12 eggs 3 cups of sugar 6 teaspoons of vanilla 18 tbsp of cocoa powder 3 cups of chocolate chips

Write you own example equation about and item that has multiple parts/pieces…

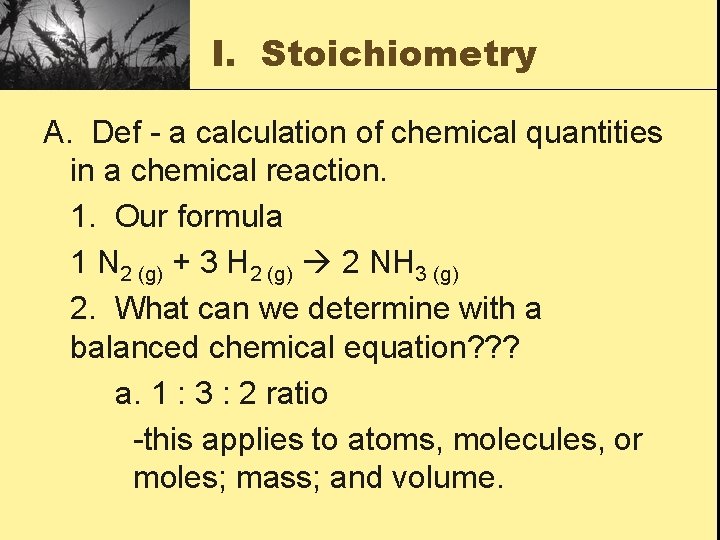
I. Stoichiometry A. Def - a calculation of chemical quantities in a chemical reaction. 1. Our formula 1 N 2 (g) + 3 H 2 (g) 2 NH 3 (g) 2. What can we determine with a balanced chemical equation? ? ? a. 1 : 3 : 2 ratio -this applies to atoms, molecules, or moles; mass; and volume.

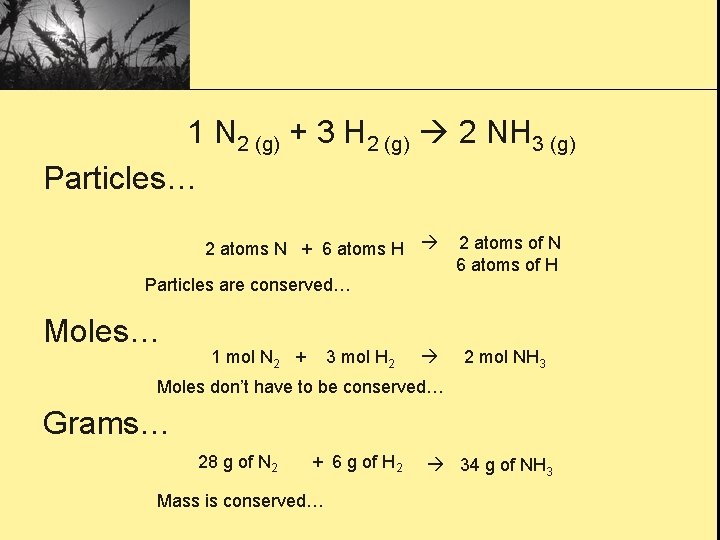
1 N 2 (g) + 3 H 2 (g) 2 NH 3 (g) Particles… 2 atoms N + 6 atoms H 2 atoms of N 6 atoms of H Particles are conserved… Moles… 1 mol N 2 + 3 mol H 2 2 mol NH 3 Moles don’t have to be conserved… Grams… 28 g of N 2 + 6 g of H 2 Mass is conserved… 34 g of NH 3

Gas/Volume (at STP)… 22. 4 L of N 2 + 67. 2 L of H 2 44. 8 L of NH 3 Volume doesn’t have to be conserved… *Mass and atoms are always conserved… *Molecules, formula units, moles, and volumes are not necessarily conserved

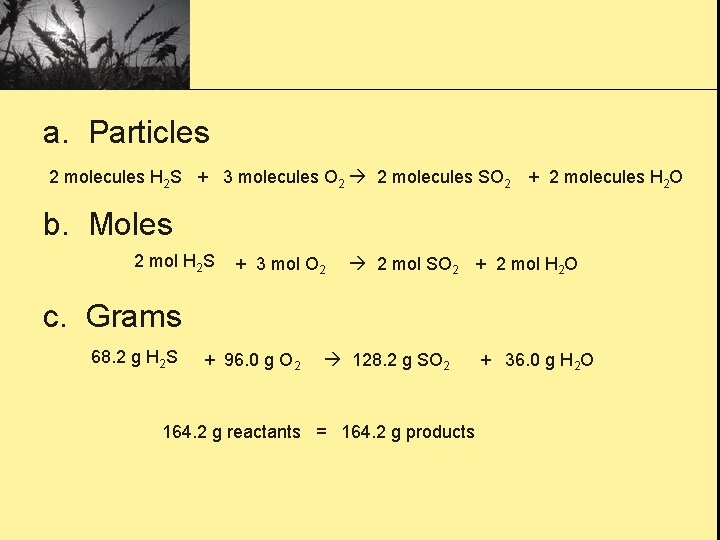
2 H 2 S(g) + 3 O 2(g) 2 SO 2(g) + 2 H 2 O(g) Hydrogen sulfide, a foul smelling gas, is found in nature in volcanic areas. The balanced chemical equation for the burning of hydrogen sulfide is given above. Interpret this equation in terms of the interaction of the three relative quantities. Do to this equation as what was on the last screen. a. Number of particles b. Number of moles c. Masses of reactants and products

a. Particles 2 molecules H 2 S + 3 molecules O 2 2 molecules SO 2 + 2 molecules H 2 O b. Moles 2 mol H 2 S + 3 mol O 2 2 mol SO 2 + 2 mol H 2 O c. Grams 68. 2 g H 2 S + 96. 0 g O 2 128. 2 g SO 2 164. 2 g reactants = 164. 2 g products + 36. 0 g H 2 O

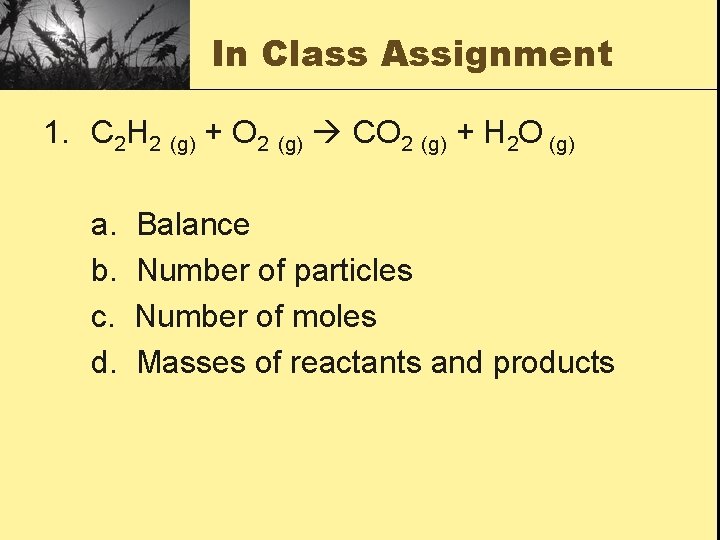
In Class Assignment 1. C 2 H 2 (g) + O 2 (g) CO 2 (g) + H 2 O (g) a. b. c. d. Balance Number of particles Number of moles Masses of reactants and products