Chemistry 111112 CHEMICAL REACTIONS Writing Chemical Reactions In





- Slides: 12

Chemistry 111/112 CHEMICAL REACTIONS

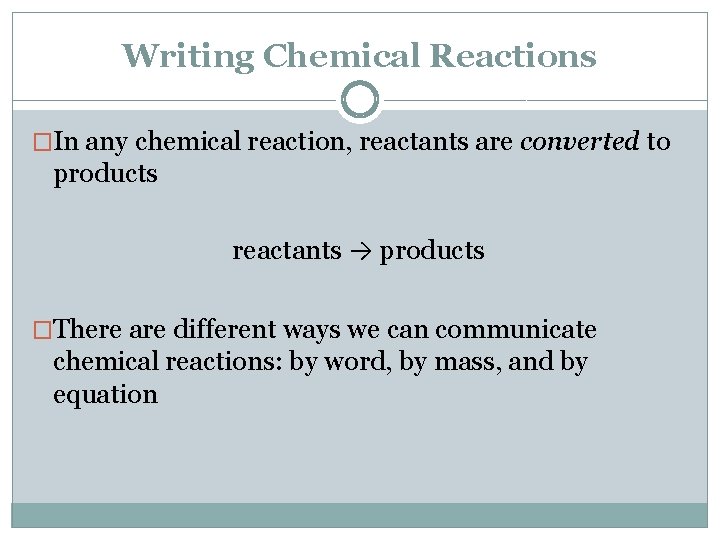
Writing Chemical Reactions �In any chemical reaction, reactants are converted to products reactants → products �There are different ways we can communicate chemical reactions: by word, by mass, and by equation

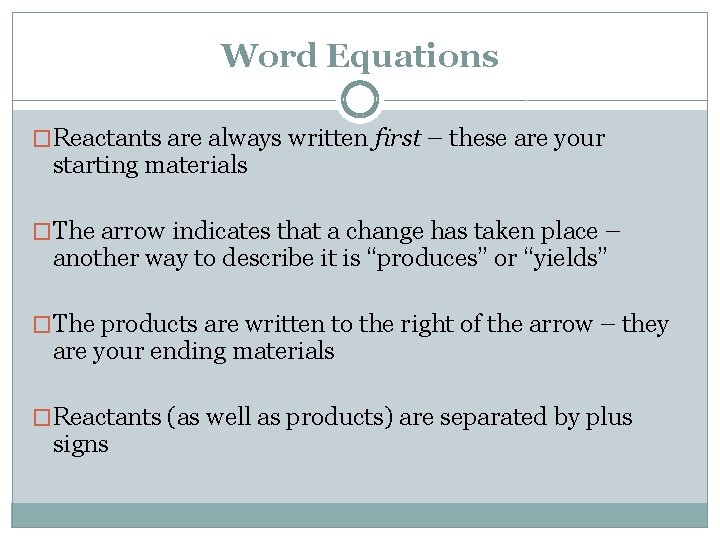
Word Equations �Reactants are always written first – these are your starting materials �The arrow indicates that a change has taken place – another way to describe it is “produces” or “yields” �The products are written to the right of the arrow – they are your ending materials �Reactants (as well as products) are separated by plus signs

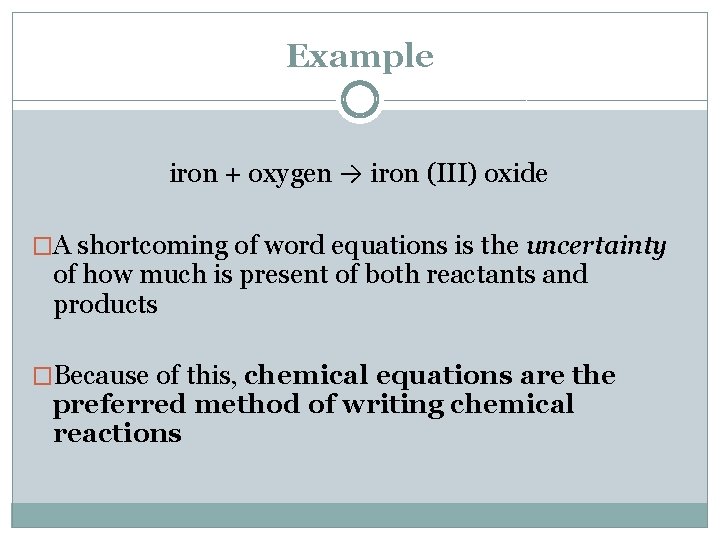
Example iron + oxygen → iron (III) oxide �A shortcoming of word equations is the uncertainty of how much is present of both reactants and products �Because of this, chemical equations are the preferred method of writing chemical reactions

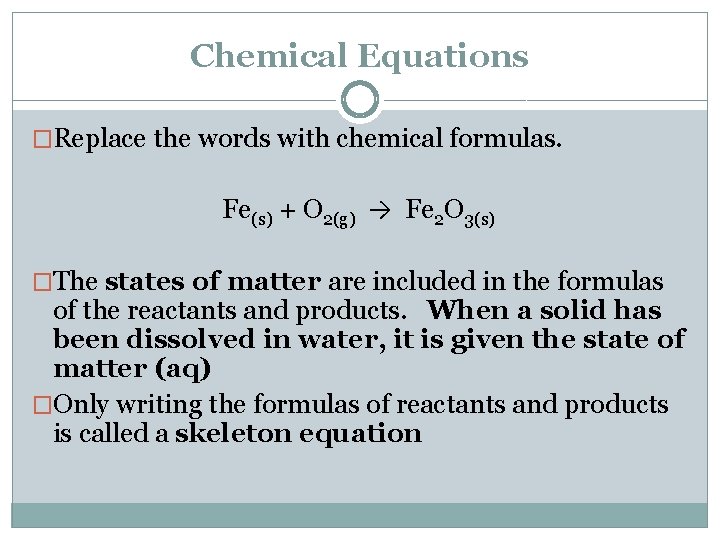
Chemical Equations �Replace the words with chemical formulas. Fe(s) + O 2(g) → Fe 2 O 3(s) �The states of matter are included in the formulas of the reactants and products. When a solid has been dissolved in water, it is given the state of matter (aq) �Only writing the formulas of reactants and products is called a skeleton equation


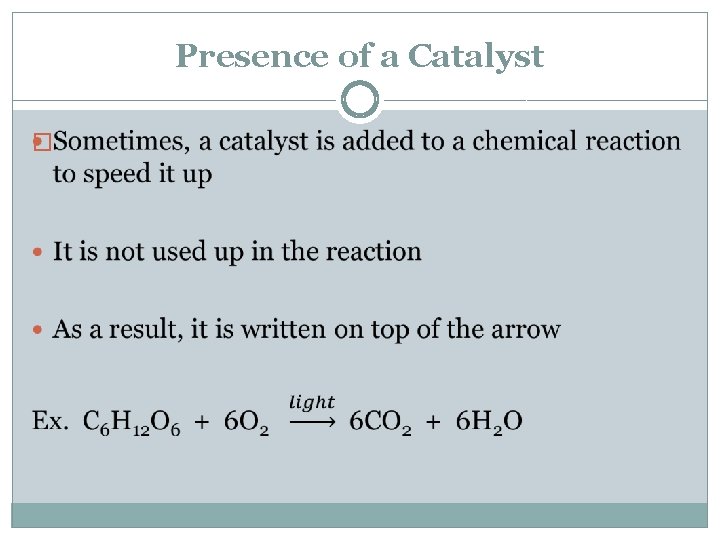
Presence of a Catalyst �

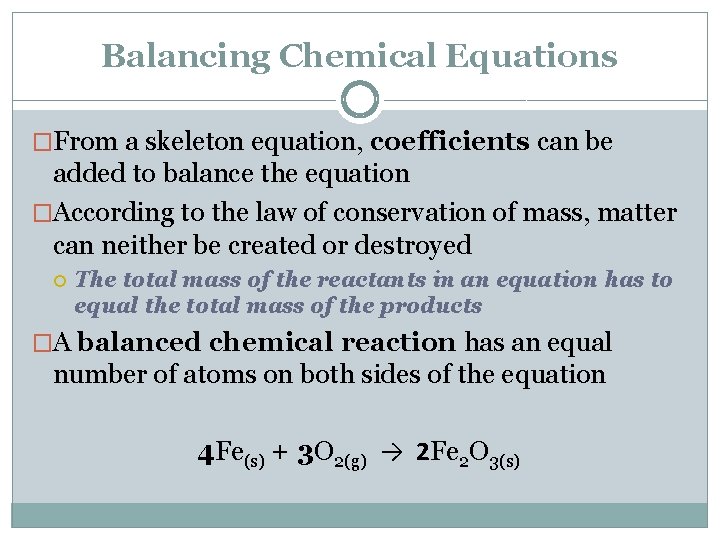
Balancing Chemical Equations �From a skeleton equation, coefficients can be added to balance the equation �According to the law of conservation of mass, matter can neither be created or destroyed The total mass of the reactants in an equation has to equal the total mass of the products �A balanced chemical reaction has an equal number of atoms on both sides of the equation 4 Fe(s) + 3 O 2(g) → 2 Fe 2 O 3(s)

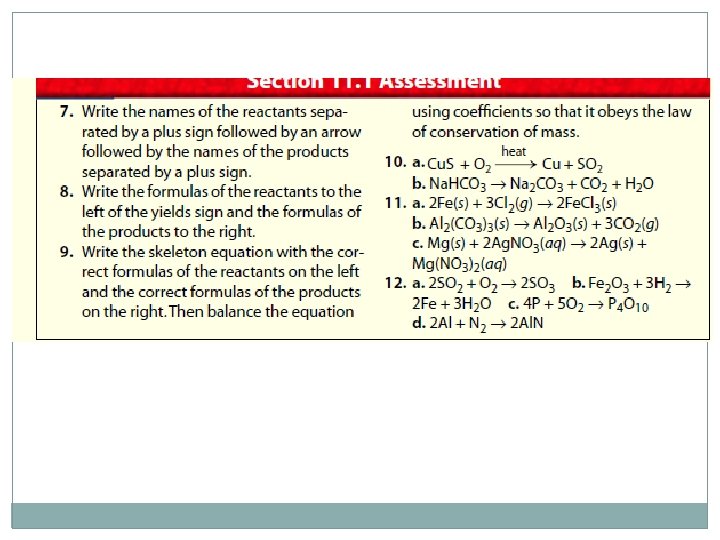
Practice �Questions 3 & 4 page 327 �Questions 5 & 6 page 328 �Questions 7 – 12 page 329 �Rewrite the following word equations as a balanced chemical equation: aluminum sulfate + calcium hydroxide → aluminum hydroxide + calcium sulfate �Rewrite the following word equation as a balanced chemical equation: phosphoric acid + sodium hydroxide → sodium phosphate + water



For the remainder of class… Complete the handout on writing word equations, skeleton equations and balancing equations - Due Complete Section 11. 1 Review - Due
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Types of reactions
Types of reactions Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Chapter 6 section 1 atoms elements and compounds answer key
Chapter 6 section 1 atoms elements and compounds answer key Section 1 chemical changes
Section 1 chemical changes Are kc and kp equal
Are kc and kp equal Redox reaction
Redox reaction Types of reactions chemistry
Types of reactions chemistry 5 types of reactions in chemistry
5 types of reactions in chemistry Type of reactions chemistry
Type of reactions chemistry