Chemistry 11 Module 4 Molecular Structure When you

















- Slides: 30

Chemistry 11 Module 4 Molecular Structure

When you want to bond, consider the Valence When atoms join to form a molecule, atoms never get close enough to each other for their nuclei to interact. To bond, you only need to deal with electrons. However, not all electrons are important to bond, but just the outside energy shell of them, or the valence electrons. Valence electrons: the electrons that exist farthest away from an atom’s nucleus. They are the electrons with the highest energy level number. **An atom’s chemistry or chemical behavior is determined mostly by the number of valence electrons it has. **

A rule you likely already know… Atoms in the same column of the periodic table have the same number of valence electrons and have very similar chemistry or chemical behavior. Now consider the elements in column 8 A of the periodic table, otherwise known as the noble gases. For each of them, the “s” and “p” orbitals in their highest energy level are full. This is a very low-energy situation, and we consider this electron configuration to be “ideal”. They don’t need to react to be more ideal, and thus, they do not react to form compounds.

More guiding principles Octet rule: Most atoms strive to attain 8 valence electrons. For atoms in groups 1 A-8 A, the number of the column in which the atom is located on the periodic table equals the number of valence electrons the atom has.

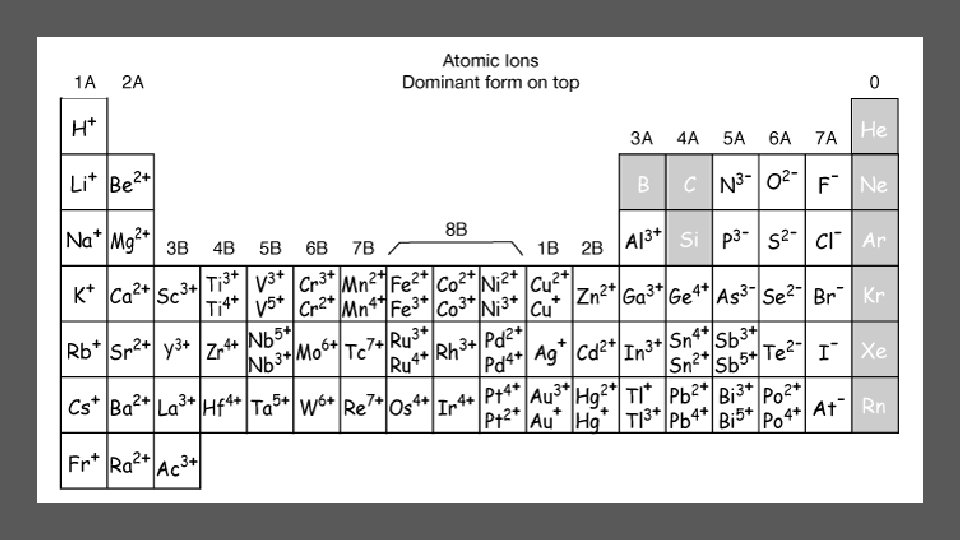
Let’s examine the Periodic Table again

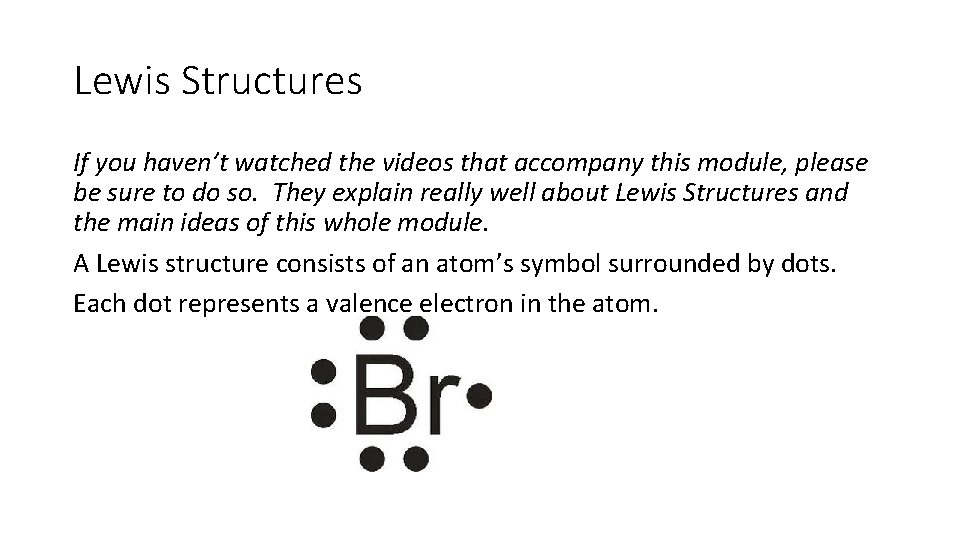
Lewis Structures If you haven’t watched the videos that accompany this module, please be sure to do so. They explain really well about Lewis Structures and the main ideas of this whole module. A Lewis structure consists of an atom’s symbol surrounded by dots. Each dot represents a valence electron in the atom.

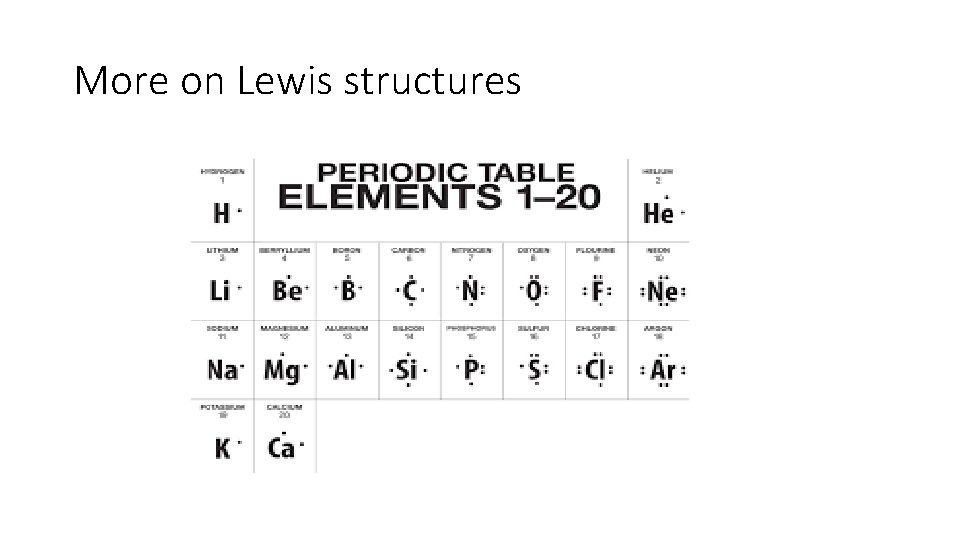
More on Lewis structures

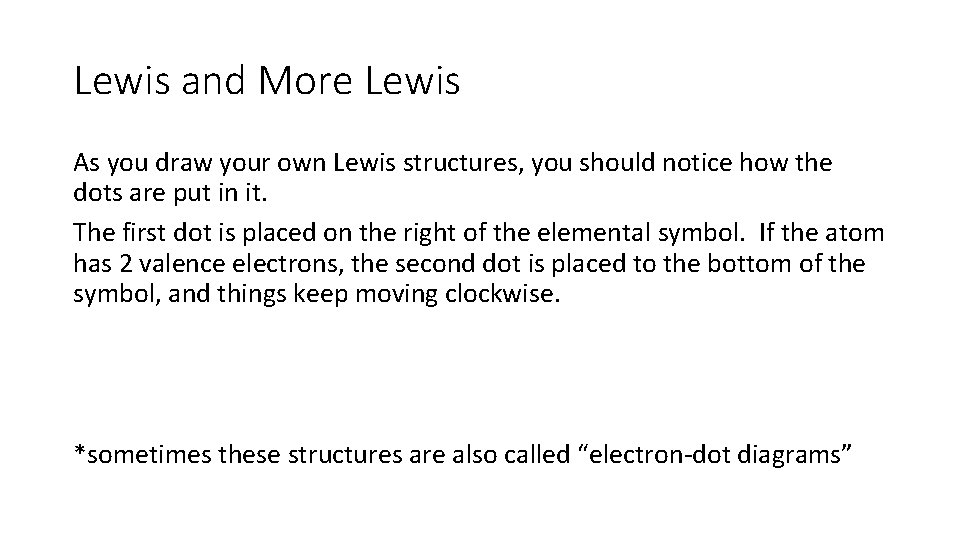
Lewis and More Lewis As you draw your own Lewis structures, you should notice how the dots are put in it. The first dot is placed on the right of the elemental symbol. If the atom has 2 valence electrons, the second dot is placed to the bottom of the symbol, and things keep moving clockwise. *sometimes these structures are also called “electron-dot diagrams”

Let’s try drawing some from On Your Own 4. 1 • A. Calcium • B. Silicon • C. Astatine

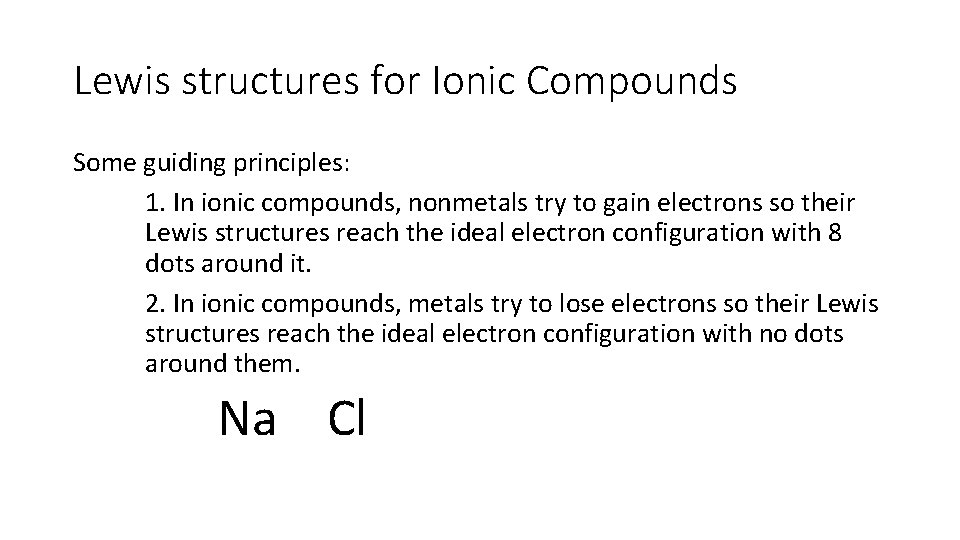
Lewis structures for Ionic Compounds Some guiding principles: 1. In ionic compounds, nonmetals try to gain electrons so their Lewis structures reach the ideal electron configuration with 8 dots around it. 2. In ionic compounds, metals try to lose electrons so their Lewis structures reach the ideal electron configuration with no dots around them. Na Cl

Ionic bonds for ions If Na loses an electron, it is no longer a sodium atom. It becomes a sodium ion. It is now “positive”, so it is called a “cation”. If Cl picks up an electron, it is no longer a chlorine atom. It becomes a chlorine ion. It is now “negative”, so it is called an “anion”. Ionic bonding like this, with ions, has a naming pattern: 1. Positive cations have the same name as the atom from which they came. 2. Negative anions add an –ide suffix to the name of the atom from which they came.

Consider a more elaborate case • Consider Mg. F 2 (p. 139 in text) Mg F

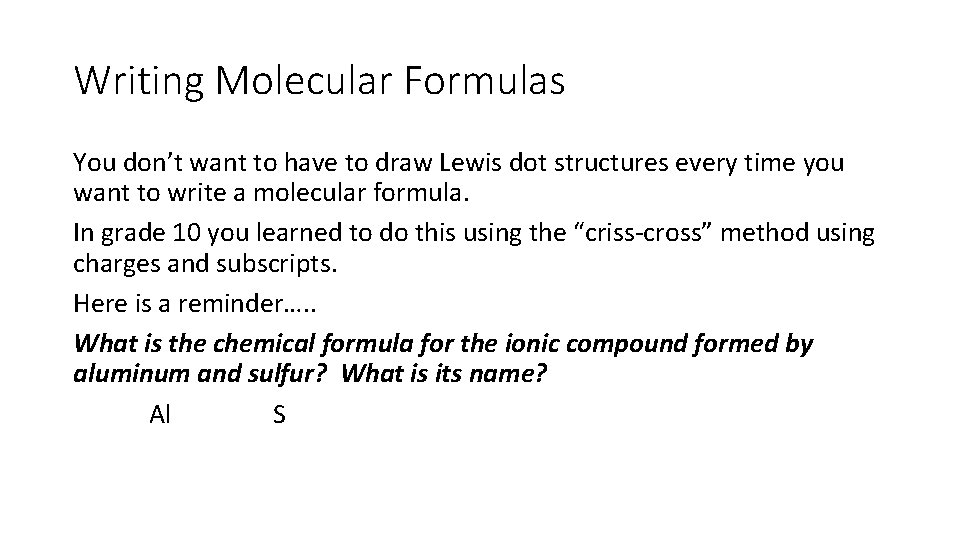
Writing Molecular Formulas You don’t want to have to draw Lewis dot structures every time you want to write a molecular formula. In grade 10 you learned to do this using the “criss-cross” method using charges and subscripts. Here is a reminder…. . What is the chemical formula for the ionic compound formed by aluminum and sulfur? What is its name? Al S

More practice, On Your Own 4. 2 and 4. 3 4. 2 Give the chemical formulas for the following compounds: a. beryllium chloride b. potassium oxide c. aluminum phosphide d. magnesium nitride 4. 3 What is the chemical formula for the compound that forms when cesium and nitrogen are reacted together? What is its name?

More difficult compounds with more than one possible valence charge Your book talks about theory behind why the transition metals in the middle of the periodic table sometimes have more than one choice for the number of valence electrons they carry. If that is interesting to you, please read it. You just need to know how to work with the options for this course, however. Remember how some different elements show that they can have different charges? ?


More About Multivalent Elements If Copper has a 2+ charge, we call it Cu(II). If it has a 1+ charge, we call it Cu(I). Remember? You have to add the Roman numeral in parentheses to reflect the charge being used. Let’s review with some examples…

On Your Own 4. 4 and 4. 5 (p. 145) 4. 4 Give the chemical formulas of the following compounds: : a. tin (IV) fluoride b. chromium (III) chloride c. copper (II) oxide 4. 5 Give the names of the following compounds: a. Fe. F 3 b. Ni. I c. Mn 2 O 3

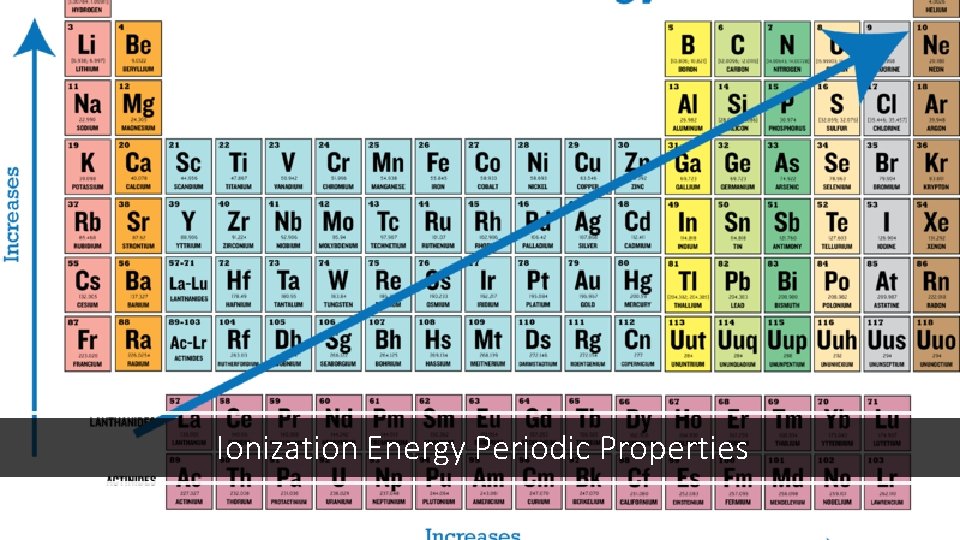
Shifting to another topic for a while… Ionization Energy and Periodic Properties Some elements on the periodic table want to ionize and bond more than others. Ionization: The process by which an atom turns into an ion by gaining or losing electrons. Ionization energy: The amount of energy needed to take an electron away from an atom. The elements on the left side of the periodic table have a low ionization energy (they give up their electrons easily), while the atoms on the right side have high ionization energies. Any atomic property that varies regularly across the periodic table is called a periodic property.

Ionization Energy Periodic Properties

Electronegativity is a measure of how strongly an atom attracts extra electrons to itself. Electronegativity follows the same periodic trend as Ionization Energy. In general, the electronegativity of atoms increases from left to right on the periodic table. It also increases from bottom to top on the periodic table.

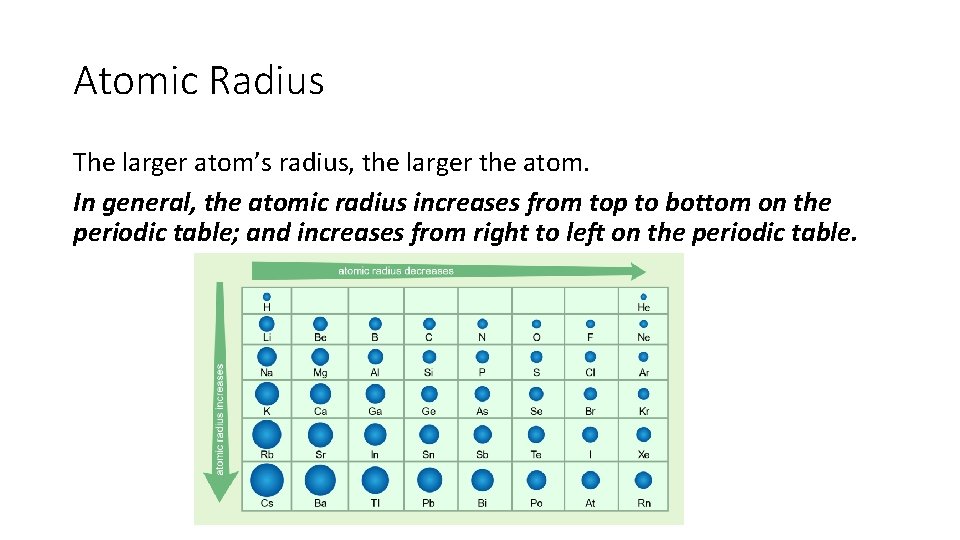
Atomic Radius The larger atom’s radius, the larger the atom. In general, the atomic radius increases from top to bottom on the periodic table; and increases from right to left on the periodic table.

On Your Own 4. 6 -4. 11 4. 6 Order the following atoms in terms of increasing ionization energy: At, Ir, Pb, Cs 4. 7 Which atom most readily gives up its electrons? Ca, Be, Ba, Ra 4. 8 Which element desires electrons most? Ca, As, K, Se 4. 9 Order the following in terms of increasing desire for electrons: Br, Cl, I, At, F 4. 10 Which element is the largest? Ca, As, K, Se 4. 11 Order the following in terms of increasing atomic radius: Br, cl, I, At, F

Lewis Structures of Covalent Compounds Remember that covalent compounds only happen with non-metals, and they share electrons instead of give them away and take them. There are some elements that always shows up in pairs when found in nature. They are called “diatomic molecules”. These are: N, O, Cl, F, Br, I, At, and H

Covalent bonds and Lewis Dots 1. Count the valence electrons in the entire molecule by adding up the valence electrons of the individual atoms. 2. Put the atom with the most unpaired electrons in the center. If there is more than on of those atoms, put them all in the center, bonded together. 3. Arrange the other atoms around the central atom and bond them with a single covalent bond. 4. Fill in the octets of the outside atoms. 5. Make sure the central atom has its octet.

Let’s Try… On Your Own 4. 12 (p. 156) 4. 12 Draw the Lewis structures for the following molecules: a. NH 3 b. Si. Br 4 c. H 2 S d. ICl

Double and Triple Bonds • Let’s consider O 2, Oxygen in its diatomic form: O O

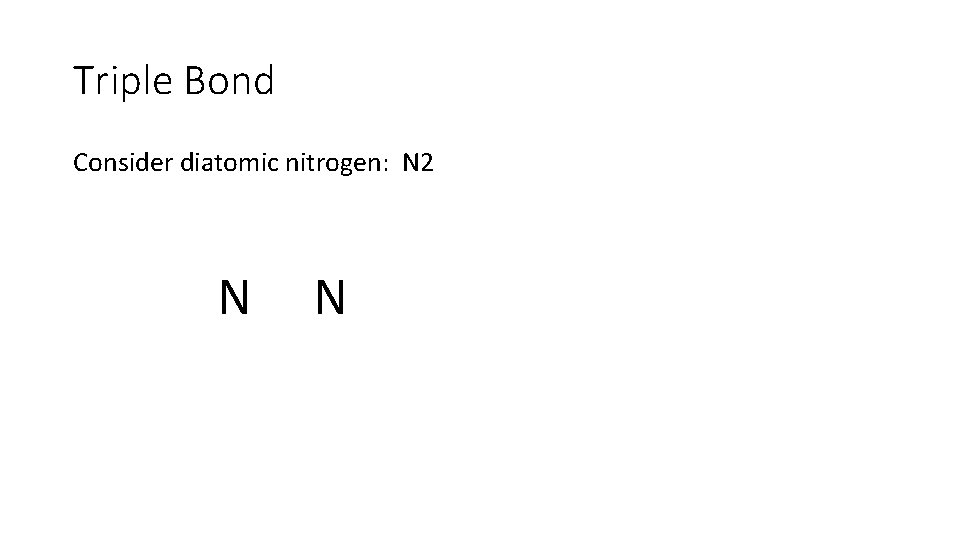
Triple Bond Consider diatomic nitrogen: N 2 N N

Last On Your Own for this Module 4. 13 Draw the Lewis structure for CH 2 O 4. 14 Draw the Lewis structure for C 2 H 2

Finishing Up Practice, Practice Finish the Study Guide questions on page 170. Get help where you need it. Finish the Practice Problems on page 171. Get help where you need it. When you feel good about what you know, take the Module 4 Exam on m. Yrcoa.