Chemistry 100 Chapter 16 Oxidation Reduction Oxidation and







- Slides: 28

Chemistry 100 Chapter 16 Oxidation & Reduction

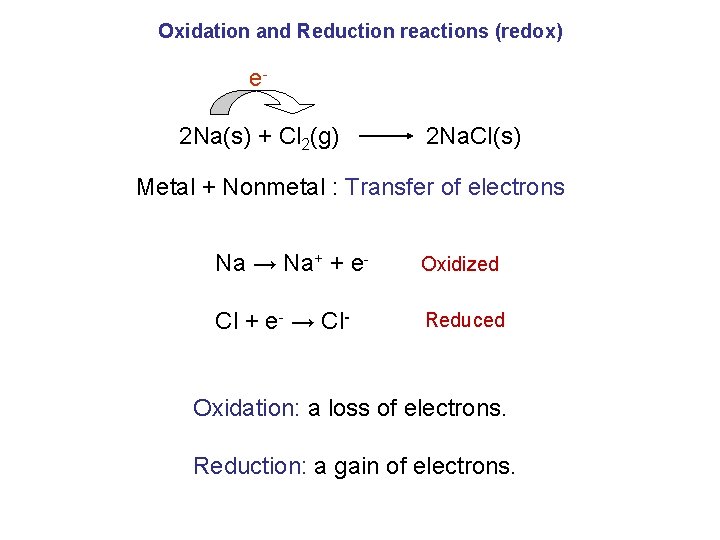
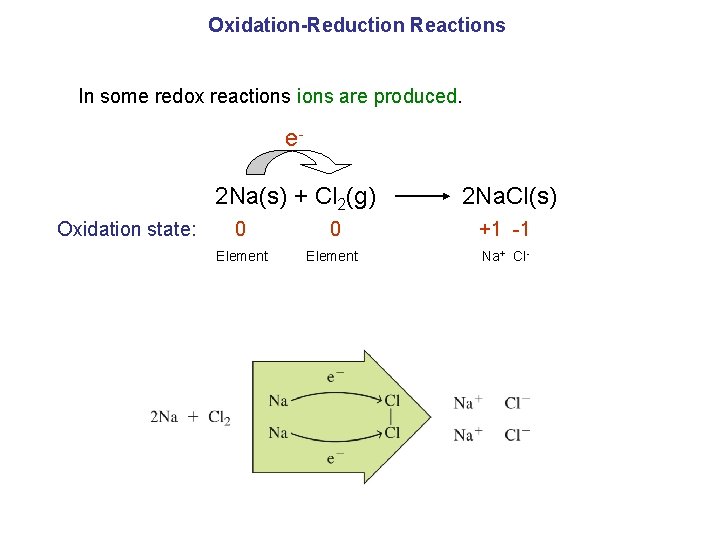
Oxidation and Reduction reactions (redox) e 2 Na(s) + Cl 2(g) 2 Na. Cl(s) Metal + Nonmetal : Transfer of electrons Na → Na+ + e- Oxidized Cl + e- → Cl- Reduced Oxidation: a loss of electrons. Reduction: a gain of electrons.

Oxidation States (Oxidation numbers) Assigning charges to the various atoms in a compound. Keep track of electrons in redox reactions.

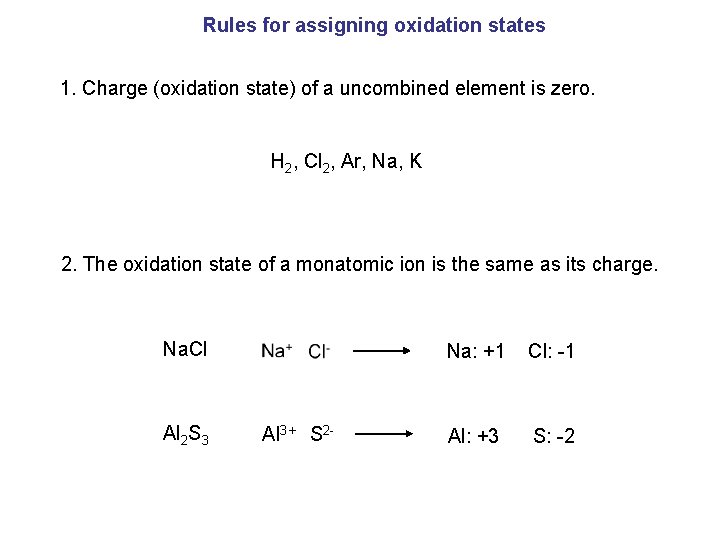
Rules for assigning oxidation states 1. Charge (oxidation state) of a uncombined element is zero. H 2, Cl 2, Ar, Na, K 2. The oxidation state of a monatomic ion is the same as its charge. Na. Cl Al 2 S 3 Na: +1 Cl: -1 Al 3+ S 2 - Al: +3 S: -2

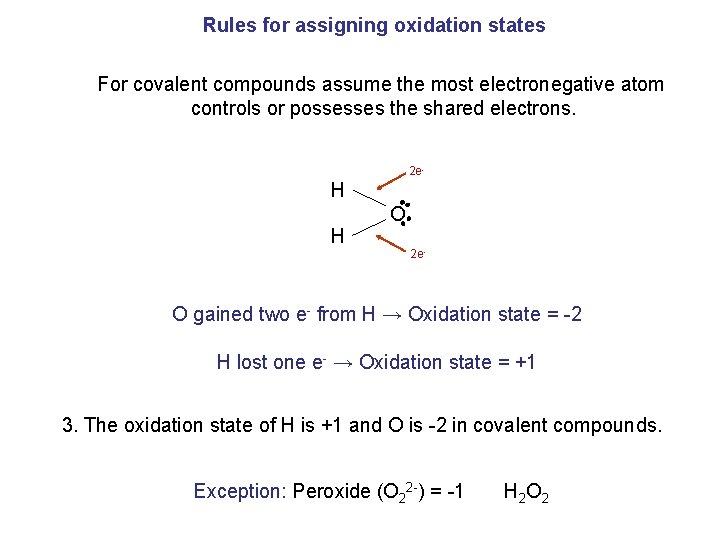
Rules for assigning oxidation states For covalent compounds assume the most electronegative atom controls or possesses the shared electrons. 2 e- H H O 2 e- O gained two e- from H → Oxidation state = -2 H lost one e- → Oxidation state = +1 3. The oxidation state of H is +1 and O is -2 in covalent compounds. Exception: Peroxide (O 22 -) = -1 H 2 O 2

Rules for assigning oxidation states 4. Sum of oxidation states = 0 in a neutral compound. SO 2 x + 2 × (-2) = 0 x + (-4) = 0 x = +4 NH 3 x + 3 × (+1) = 0 x + (+3) = 0 N = -3

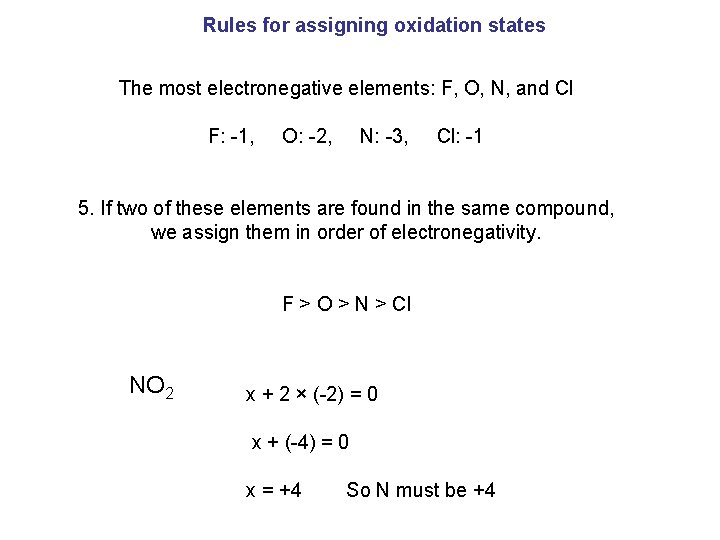
Rules for assigning oxidation states The most electronegative elements: F, O, N, and Cl F: -1, O: -2, N: -3, Cl: -1 5. If two of these elements are found in the same compound, we assign them in order of electronegativity. F ˃ O ˃ N ˃ Cl NO 2 x + 2 × (-2) = 0 x + (-4) = 0 x = +4 So N must be +4

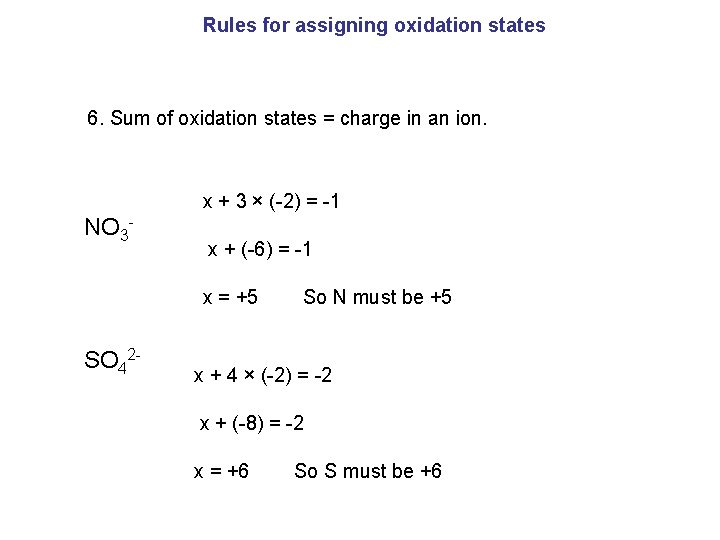
Rules for assigning oxidation states 6. Sum of oxidation states = charge in an ion. x + 3 × (-2) = -1 NO 3 - x + (-6) = -1 x = +5 So N must be +5 SO 42 - x + 4 × (-2) = -2 x + (-8) = -2 x = +6 So S must be +6

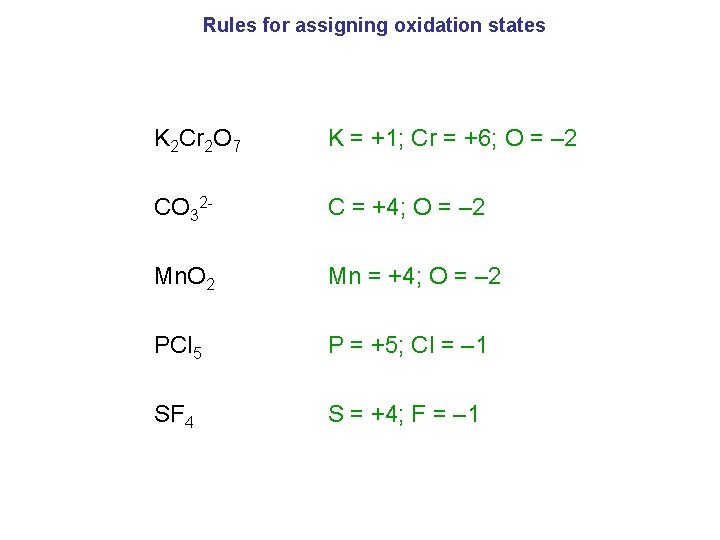
Rules for assigning oxidation states K 2 Cr 2 O 7 K = +1; Cr = +6; O = – 2 CO 32 - C = +4; O = – 2 Mn. O 2 Mn = +4; O = – 2 PCl 5 P = +5; Cl = – 1 SF 4 S = +4; F = – 1

Oxidation-Reduction Reactions In some redox reactions are produced. e 2 Na(s) + Cl 2(g) 2 Na. Cl(s) Oxidation state: 0 +1 -1 Element Na + Cl-

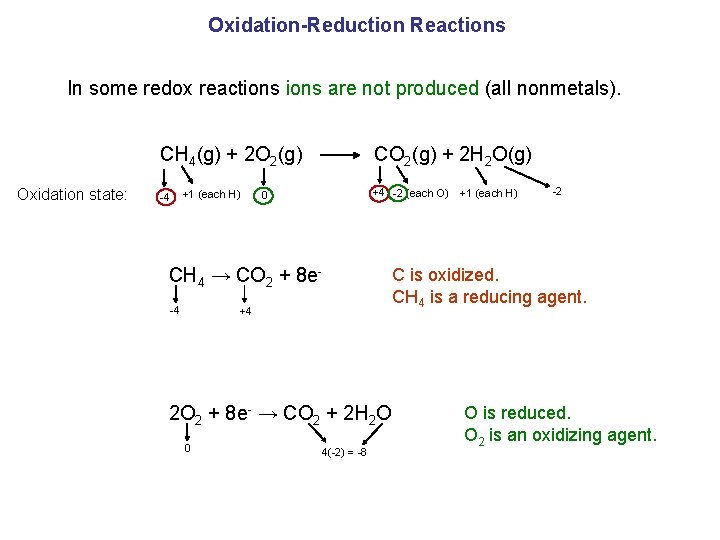
Oxidation-Reduction Reactions In some redox reactions are not produced (all nonmetals). CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) Oxidation state: -4 +1 (each H) +4 -2 (each O) 0 CH 4 → CO 2 + 8 e-4 +4 2 O 2 + 8 e- → CO 2 + 2 H 2 O 0 4(-2) = -8 +1 (each H) -2 C is oxidized. CH 4 is a reducing agent. O is reduced. O 2 is an oxidizing agent.

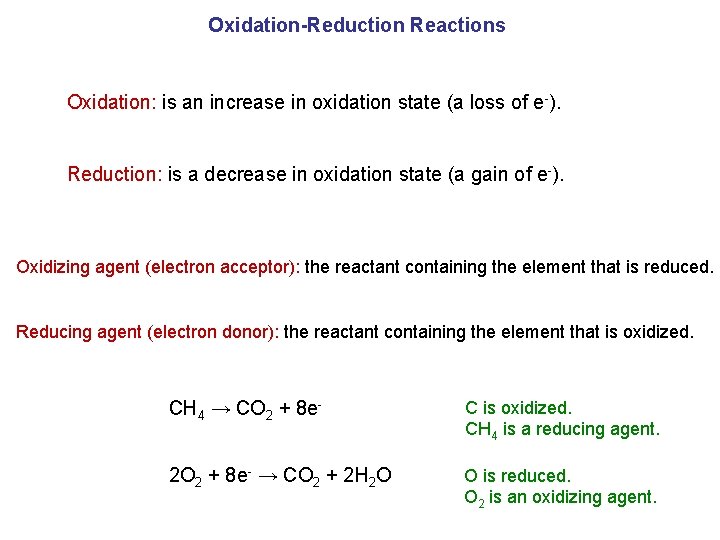
Oxidation-Reduction Reactions Oxidation: is an increase in oxidation state (a loss of e-). Reduction: is a decrease in oxidation state (a gain of e-). Oxidizing agent (electron acceptor): the reactant containing the element that is reduced. Reducing agent (electron donor): the reactant containing the element that is oxidized. CH 4 → CO 2 + 8 e- C is oxidized. CH 4 is a reducing agent. 2 O 2 + 8 e- → CO 2 + 2 H 2 O O is reduced. O 2 is an oxidizing agent.

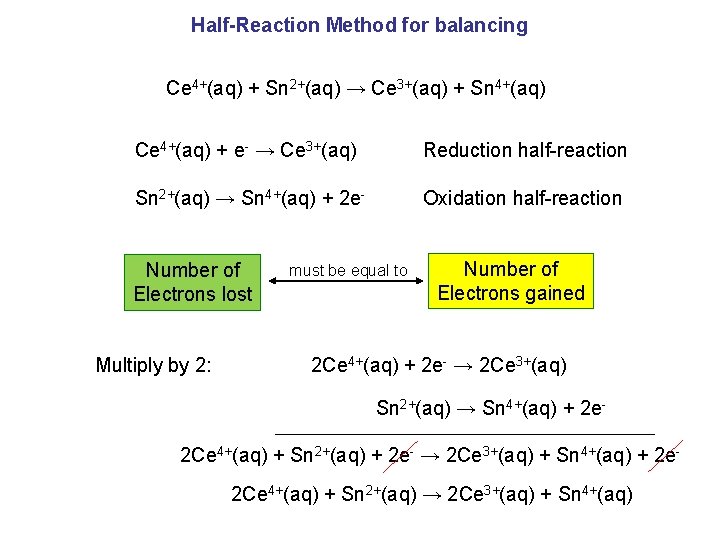
Half-Reaction Method for balancing Ce 4+(aq) + Sn 2+(aq) → Ce 3+(aq) + Sn 4+(aq) Ce 4+(aq) + e- → Ce 3+(aq) Reduction half-reaction Sn 2+(aq) → Sn 4+(aq) + 2 e- Oxidation half-reaction Number of Electrons lost Multiply by 2: must be equal to Number of Electrons gained 2 Ce 4+(aq) + 2 e- → 2 Ce 3+(aq) Sn 2+(aq) → Sn 4+(aq) + 2 e- 2 Ce 4+(aq) + Sn 2+(aq) + 2 e- → 2 Ce 3+(aq) + Sn 4+(aq) + 2 e 2 Ce 4+(aq) + Sn 2+(aq) → 2 Ce 3+(aq) + Sn 4+(aq)

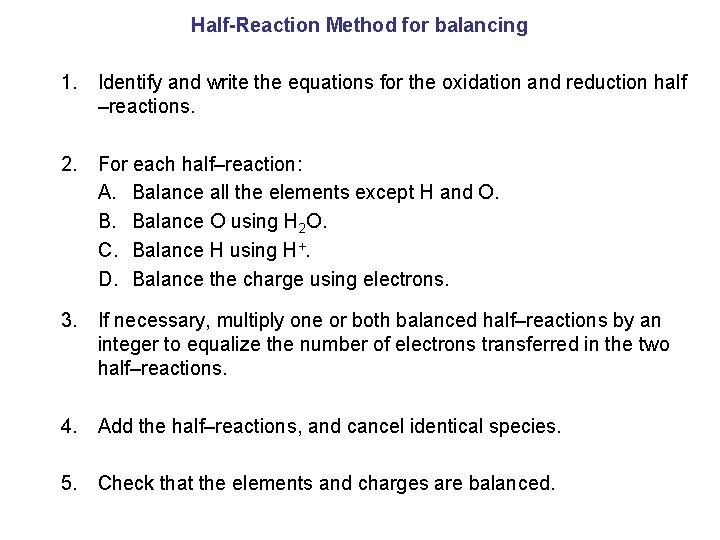
Half-Reaction Method for balancing 1. Identify and write the equations for the oxidation and reduction half –reactions. 2. For each half–reaction: A. Balance all the elements except H and O. B. Balance O using H 2 O. C. Balance H using H+. D. Balance the charge using electrons. 3. If necessary, multiply one or both balanced half–reactions by an integer to equalize the number of electrons transferred in the two half–reactions. 4. Add the half–reactions, and cancel identical species. 5. Check that the elements and charges are balanced.

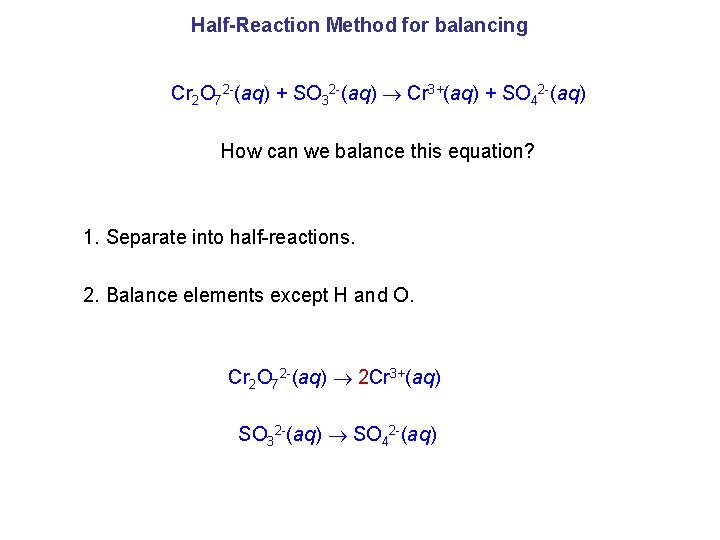
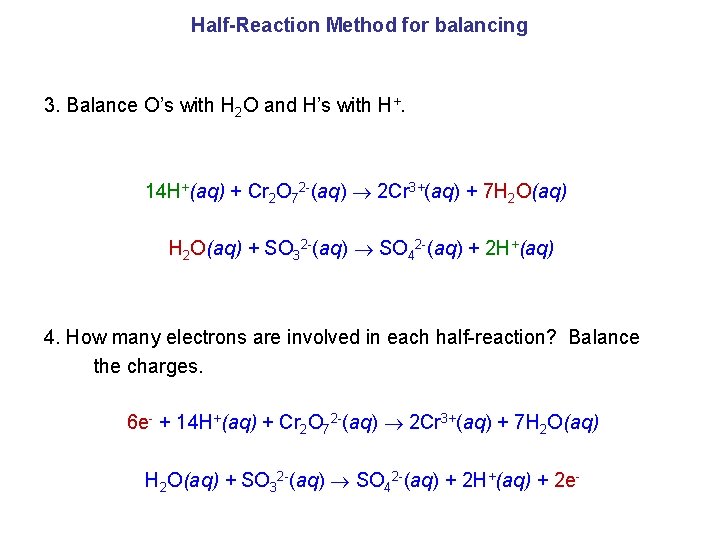
Half-Reaction Method for balancing Cr 2 O 72 -(aq) + SO 32 -(aq) Cr 3+(aq) + SO 42 -(aq) How can we balance this equation? 1. Separate into half-reactions. 2. Balance elements except H and O. Cr 2 O 72 -(aq) 2 Cr 3+(aq) SO 32 -(aq) SO 42 -(aq)

Half-Reaction Method for balancing 3. Balance O’s with H 2 O and H’s with H+. 14 H+(aq) + Cr 2 O 72 -(aq) 2 Cr 3+(aq) + 7 H 2 O(aq) + SO 32 -(aq) SO 42 -(aq) + 2 H+(aq) 4. How many electrons are involved in each half-reaction? Balance the charges. 6 e- + 14 H+(aq) + Cr 2 O 72 -(aq) 2 Cr 3+(aq) + 7 H 2 O(aq) + SO 32 -(aq) SO 42 -(aq) + 2 H+(aq) + 2 e-

Half-Reaction Method for balancing 5. Multiply whole reactions by a whole number to make the number of electrons gained equal the number of electrons lost. 6 e- + 14 H+(aq) + Cr 2 O 72 -(aq) 2 Cr 3+(aq) + 7 H 2 O(aq) 3 × ( H 2 O(aq) + SO 32 -(aq) SO 42 -(aq) + 2 H+(aq) + 2 e- ) 6. Combine half-reactions cancelling out those reactants and products that are the same on both sides, especially the electrons. 8 4 + 23+ 6 e- + 14 H (aq) + Cr 2 O 7 (aq) 2 Cr (aq) + 7 H 2 O(aq) 3 H 2 O(aq) + 3 SO 32 -(aq) 3 SO 42 -(aq) + 6 H+(aq) + 6 e. Cr 2 O 72 - + 3 SO 32 - + 8 H+ 2 Cr 3+ + 3 SO 42 - + 4 H 2 O

Electrochemistry Galvanic Cell

Electrochemistry Voltmeter Zn Al Zn 2+ Al 3+ NO 3 - Zn(NO 3)2 Solution NO 3 Al(NO 3)3 Solution

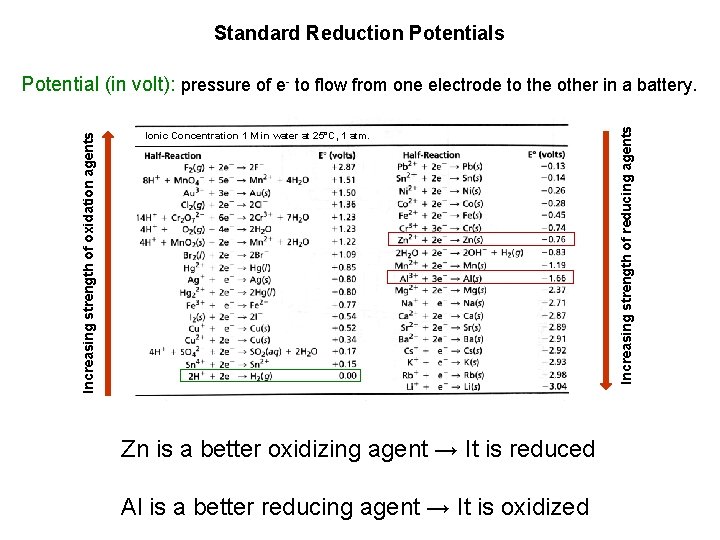
Standard Reduction Potentials Ionic Concentration 1 M in water at 25°C, 1 atm. Zn is a better oxidizing agent → It is reduced Al is a better reducing agent → It is oxidized Increasing strength of reducing agents Increasing strength of oxidation agents Potential (in volt): pressure of e- to flow from one electrode to the other in a battery.

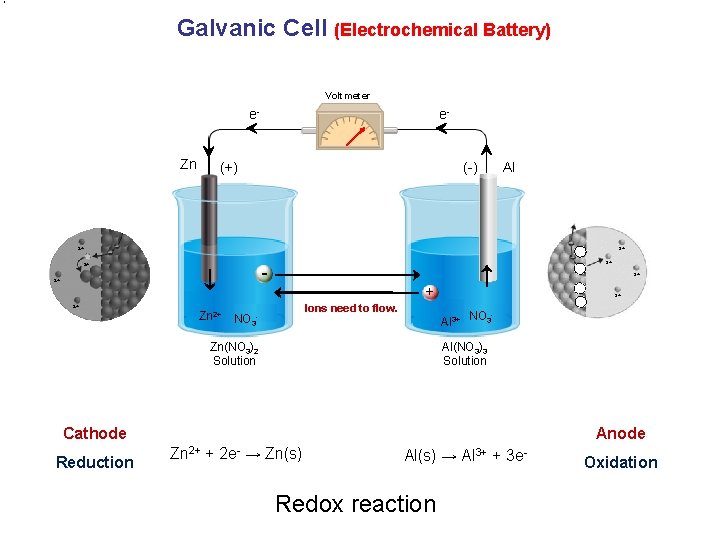
Galvanic Cell (Electrochemical Battery) Voltmeter e. Zn e- (+) (-) Al 2+ 3+ - 2+ 3+ + 2+ Ions need to flow. Zn 2+ NO 3 - 3+ Al 3+ NO 3 Zn(NO 3)2 Solution Al(NO 3)3 Solution Cathode Reduction Anode Zn 2+ + 2 e- → Zn(s) Al(s) → Al 3+ + 3 e- Redox reaction Oxidation

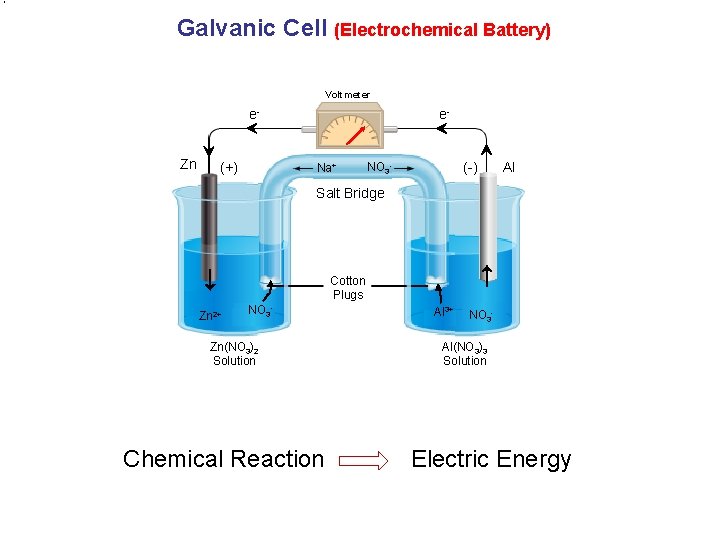
Galvanic Cell (Electrochemical Battery) Voltmeter e. Zn (+) e- Na+ (-) NO 3 - Al Salt Bridge Cotton Plugs Zn 2+ NO 3 - Zn(NO 3)2 Solution Chemical Reaction Al 3+ NO 3 - Al(NO 3)3 Solution Electric Energy

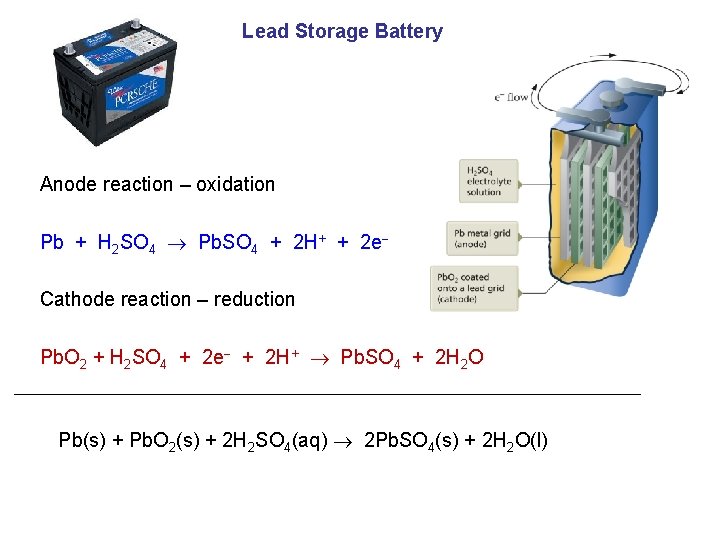
Lead Storage Battery Anode reaction – oxidation Pb + H 2 SO 4 Pb. SO 4 + 2 H+ + 2 e Cathode reaction – reduction Pb. O 2 + H 2 SO 4 + 2 e + 2 H+ Pb. SO 4 + 2 H 2 O Pb(s) + Pb. O 2(s) + 2 H 2 SO 4(aq) 2 Pb. SO 4(s) + 2 H 2 O(l)

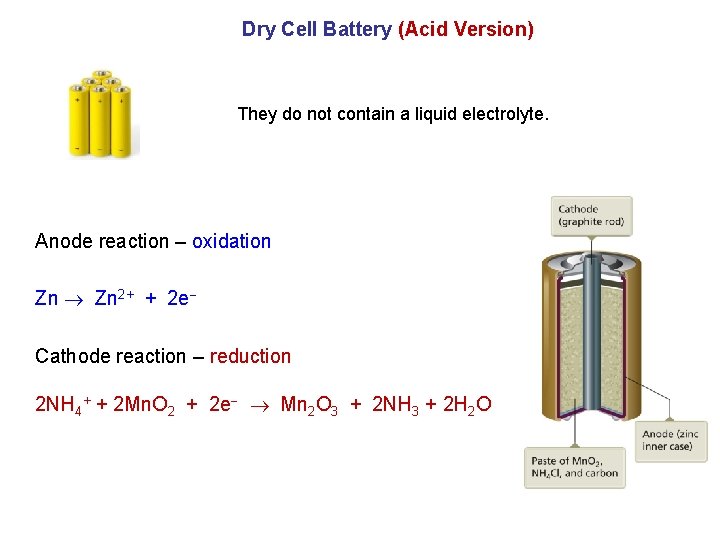
Dry Cell Battery (Acid Version) They do not contain a liquid electrolyte. Anode reaction – oxidation Zn 2+ + 2 e Cathode reaction – reduction 2 NH 4+ + 2 Mn. O 2 + 2 e Mn 2 O 3 + 2 NH 3 + 2 H 2 O

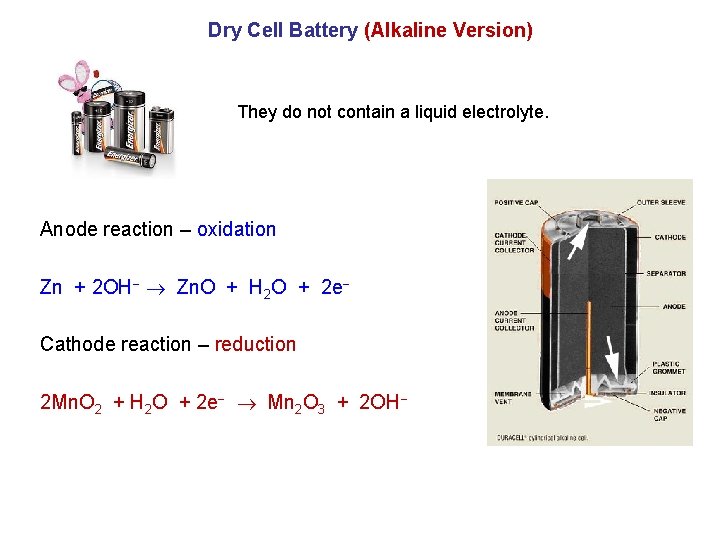
Dry Cell Battery (Alkaline Version) They do not contain a liquid electrolyte. Anode reaction – oxidation Zn + 2 OH Zn. O + H 2 O + 2 e Cathode reaction – reduction 2 Mn. O 2 + H 2 O + 2 e Mn 2 O 3 + 2 OH

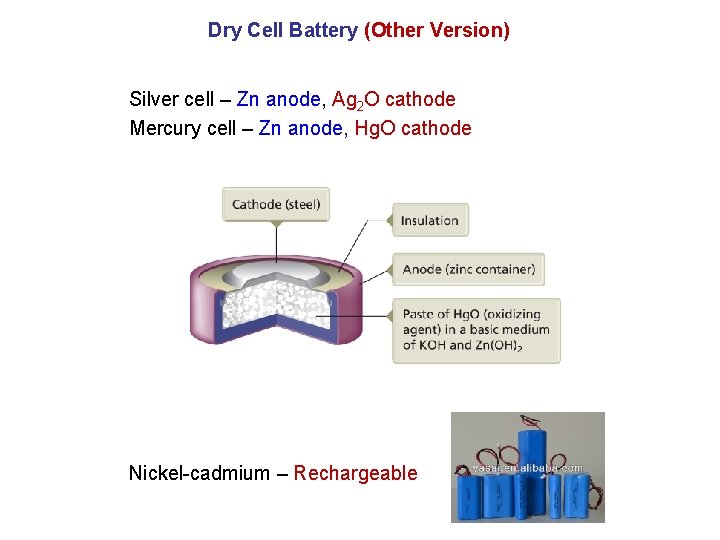
Dry Cell Battery (Other Version) Silver cell – Zn anode, Ag 2 O cathode Mercury cell – Zn anode, Hg. O cathode Nickel-cadmium – Rechargeable

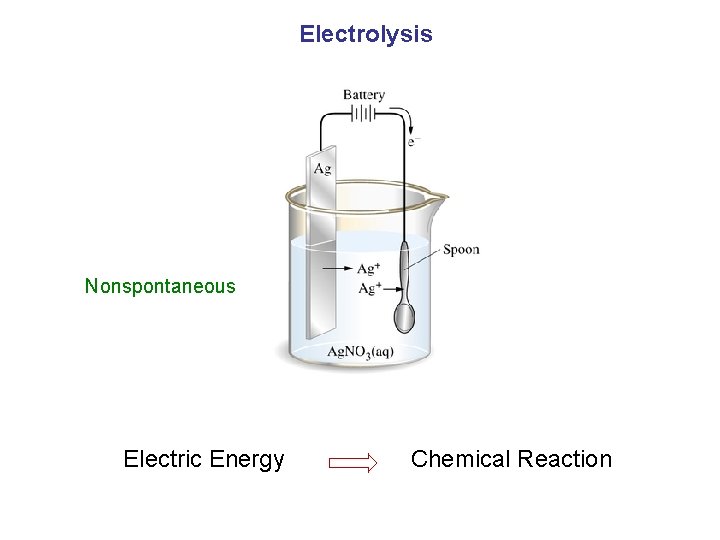
Electrolysis Nonspontaneous Electric Energy Chemical Reaction

Electrolysis O 2 H 2 O 2 H 2 O(l) Forced electric current 2 H 2(g) + O 2(g)