Chemistry 1 Notes 4 b Chapter 12 Part

Chemistry 1 Notes #4 b Chapter 12 Part II 20 December 2021
Limiting Reactant The limiting reactant is the reactant that: • is completely consumed • yields the LEAST amount of product

Calculations dealing with Limiting Reactants If amounts are given for more than one reactant, you’ve got a limiting reactant problem! Strategy: • calculate the amount of product (in mols) you could produce for each reactant • the lowest yield is theoretical yield • the reactant giving lowest yield is the limiting reactant.
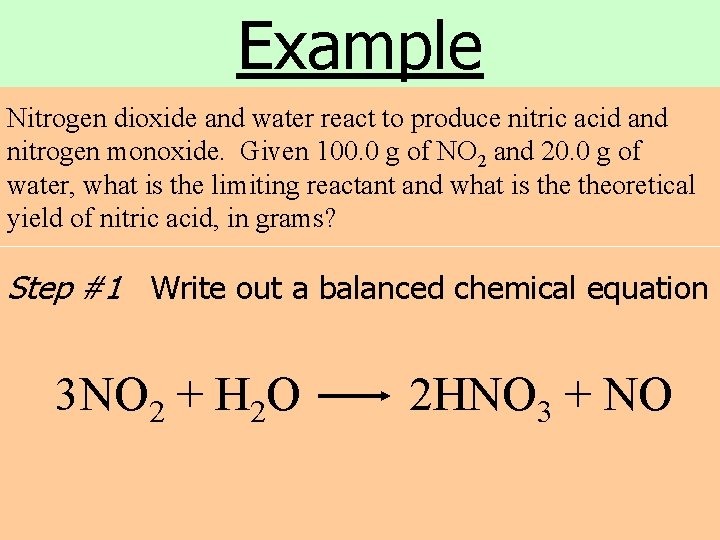
Example Nitrogen dioxide and water react to produce nitric acid and nitrogen monoxide. Given 100. 0 g of NO 2 and 20. 0 g of water, what is the limiting reactant and what is theoretical yield of nitric acid, in grams? Step #1 Write out a balanced chemical equation 3 NO 2 + H 2 O 2 HNO 3 + NO
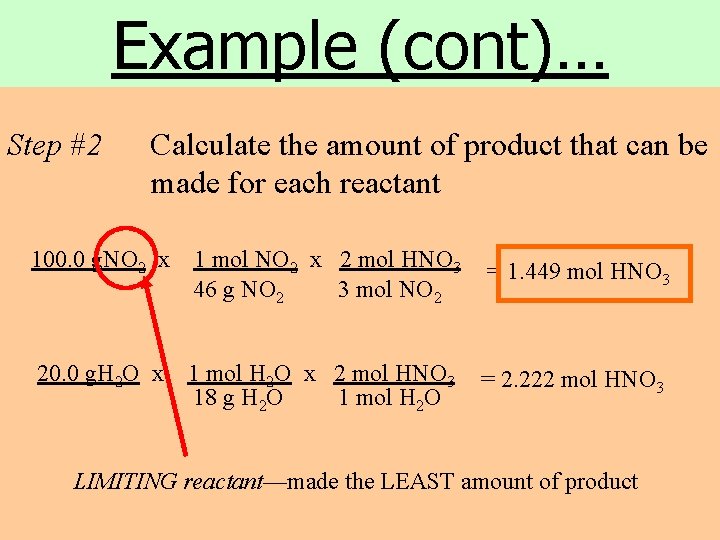
Example (cont)… Step #2 Calculate the amount of product that can be made for each reactant 100. 0 g. NO 2 x 1 mol NO 2 x 2 mol HNO 3 46 g NO 2 3 mol NO 2 = 1. 449 mol HNO 3 20. 0 g. H 2 O x 1 mol H 2 O x 2 mol HNO 3 18 g H 2 O 1 mol H 2 O = 2. 222 mol HNO 3 LIMITING reactant—made the LEAST amount of product
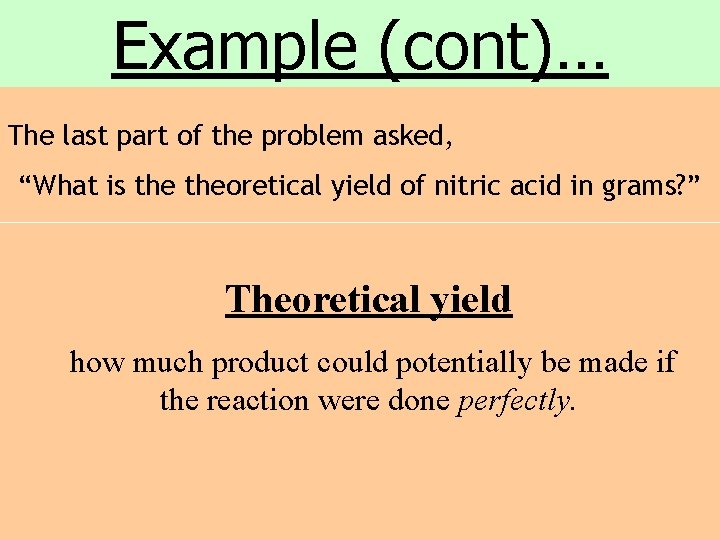
Example (cont)… The last part of the problem asked, “What is theoretical yield of nitric acid in grams? ” Theoretical yield how much product could potentially be made if the reaction were done perfectly.

Example (cont)… 1. 449 mol HNO 3 x 63 g HNO 3 1 mol HNO 3 = 91. 29 g HNO 3
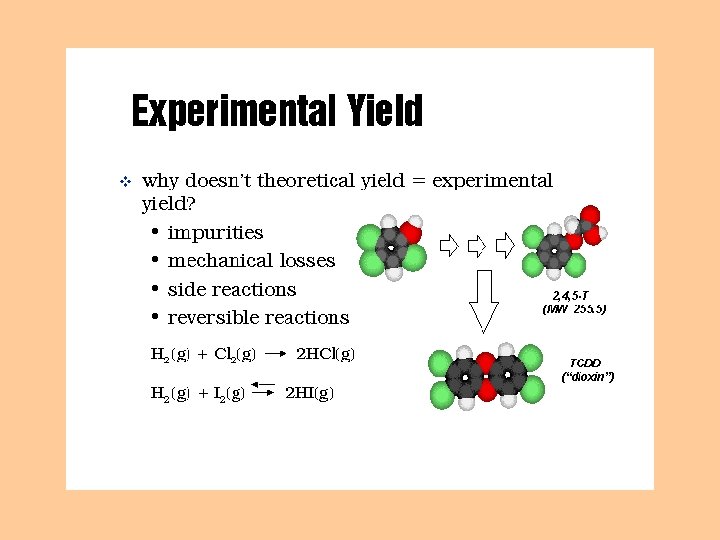

Percent Yield % yield = actual yield (experimental) theoretical yield X 100% What percent of the perfect amount did you obtain (taking into account human/mechanical error)
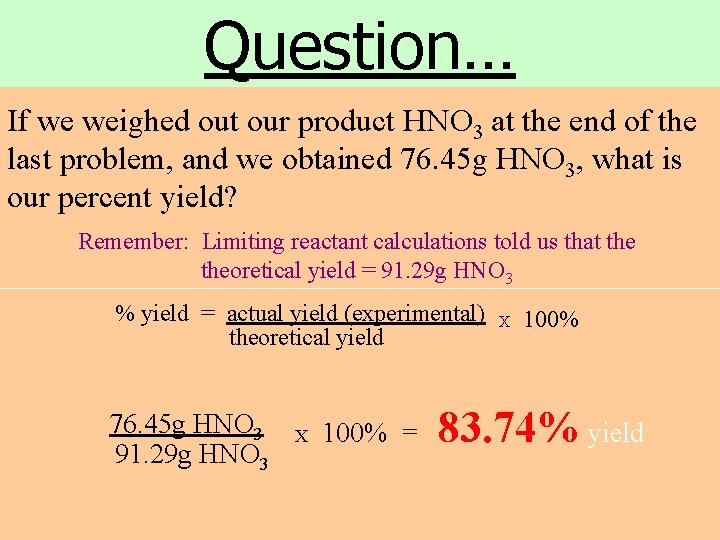
Question… If we weighed out our product HNO 3 at the end of the last problem, and we obtained 76. 45 g HNO 3, what is our percent yield? Remember: Limiting reactant calculations told us that theoretical yield = 91. 29 g HNO 3 % yield = actual yield (experimental) theoretical yield 76. 45 g HNO 3 91. 29 g HNO 3 X 100% = X 100% 83. 74% yield
- Slides: 10