Chemistry 1 Notes 3 b Chapter 11 Section

Chemistry 1 Notes # 3 b Chapter 11 Section 1 THE MOLE & particle/mass calculations Last updated 12 December 2021

MOLE • Moles- SI unit for the amount of a substance.
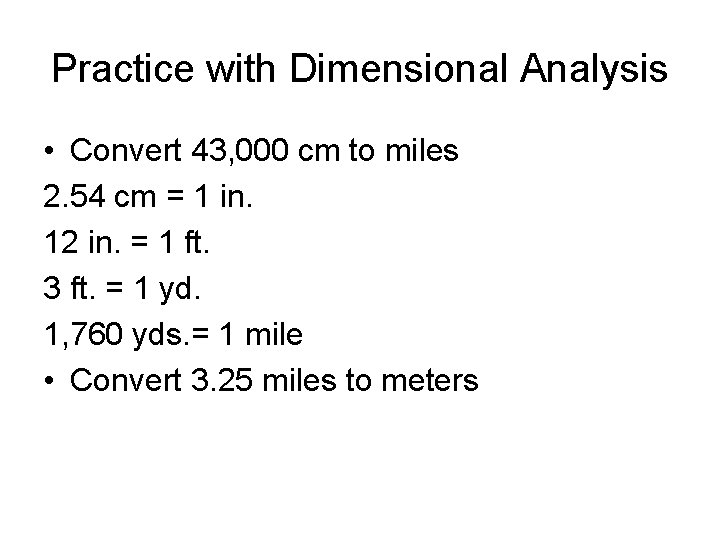
Practice with Dimensional Analysis • Convert 43, 000 cm to miles 2. 54 cm = 1 in. 12 in. = 1 ft. 3 ft. = 1 yd. 1, 760 yds. = 1 mile • Convert 3. 25 miles to meters

Measuring Matter • What is a mole? – The Mole is an SI base unit for the amount of a substance. – 6. 02 x 1023 = number of representative particles in exactly 12 g of Carbon-12 = 1 mole

• Whereas a dozen is known as 12 of something, a pair is always 2, and a gross is always 144. This number never changes but what is counted does. • The same can be said about the mole

• The mole represents 6. 02 x 1023 of something. • This number is called Avogadro’s number • In chemistry the mole is used to measure how many atoms, particles or molecules something contains

Converting Moles to Particles and Particles to moles • Using the mole and knowing how many particles, atoms or molecules, converting from the mole to how many particles and back is simple • Problem: How many atoms are in 1. 76 mol of Zn
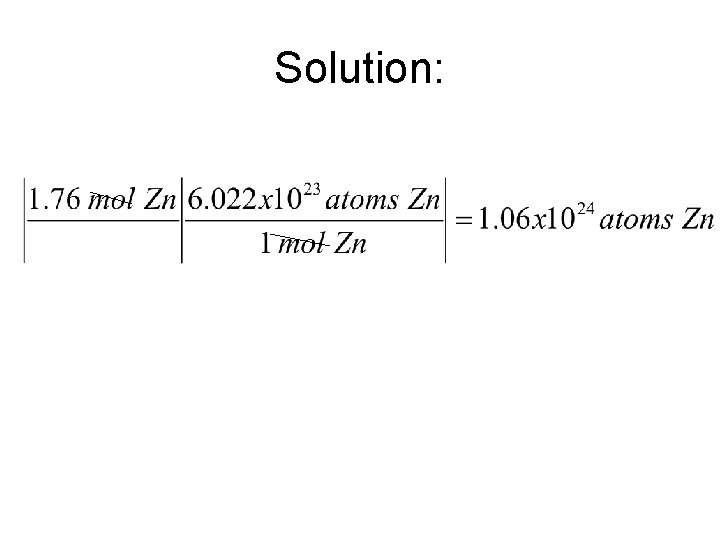
Solution:
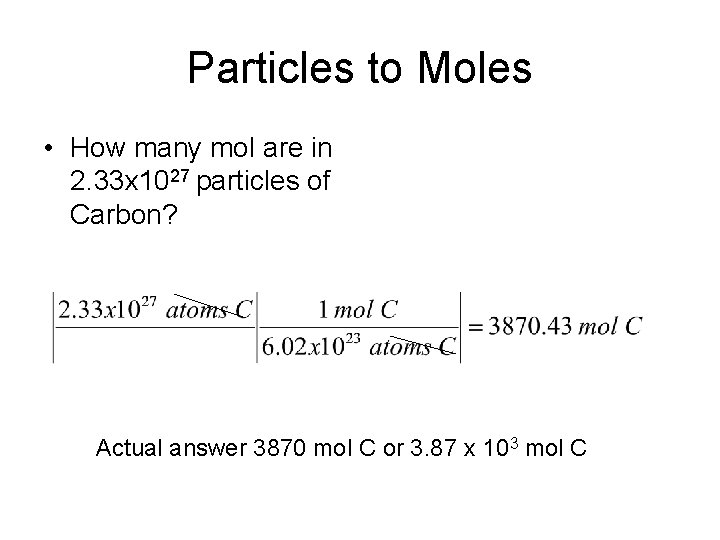
Particles to Moles • How many mol are in 2. 33 x 1027 particles of Carbon? Actual answer 3870 mol C or 3. 87 x 103 mol C
11. 2 Mass and the Mole • Molar mass the mass in grams of one mole of any substance. • For example: The atomic mass of carbon is 12. 01 amu (atomic mass units) therefore the molar mass of carbong is 12. 01 grams • What is the molar mass of Potassium?
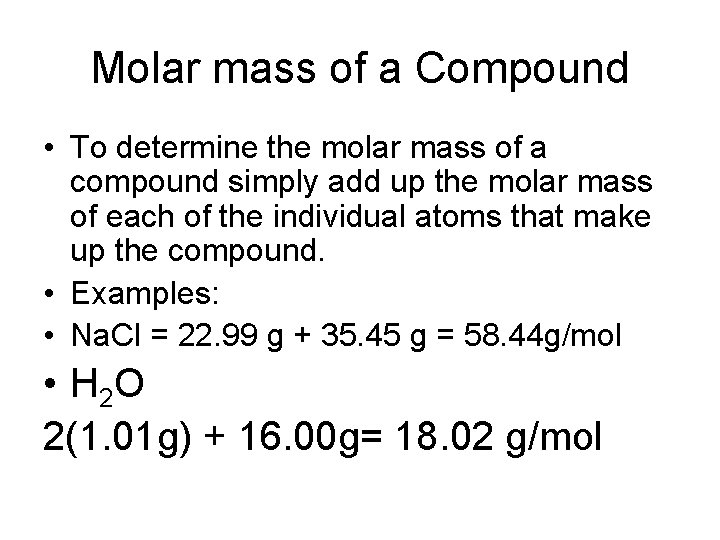
Molar mass of a Compound • To determine the molar mass of a compound simply add up the molar mass of each of the individual atoms that make up the compound. • Examples: • Na. Cl = 22. 99 g + 35. 45 g = 58. 44 g/mol • H 2 O 2(1. 01 g) + 16. 00 g= 18. 02 g/mol
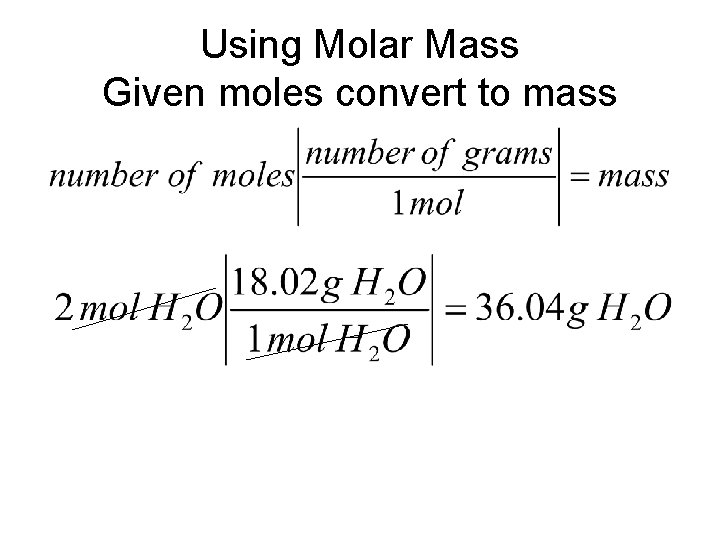
Using Molar Mass Given moles convert to mass
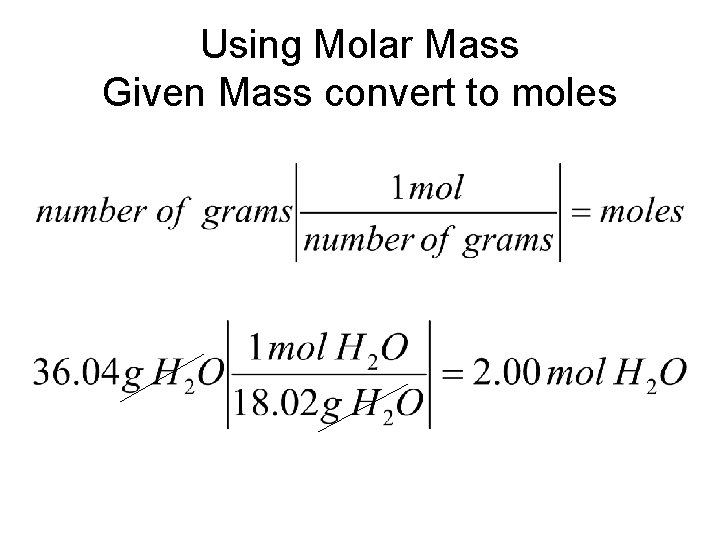
Using Molar Mass Given Mass convert to moles
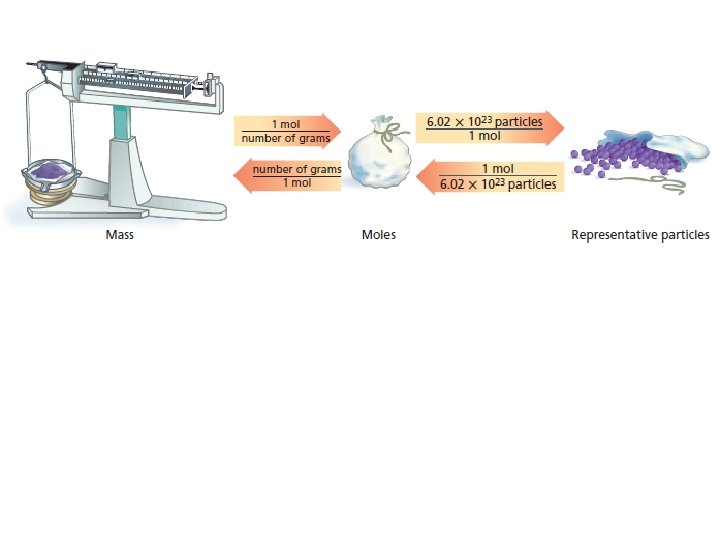
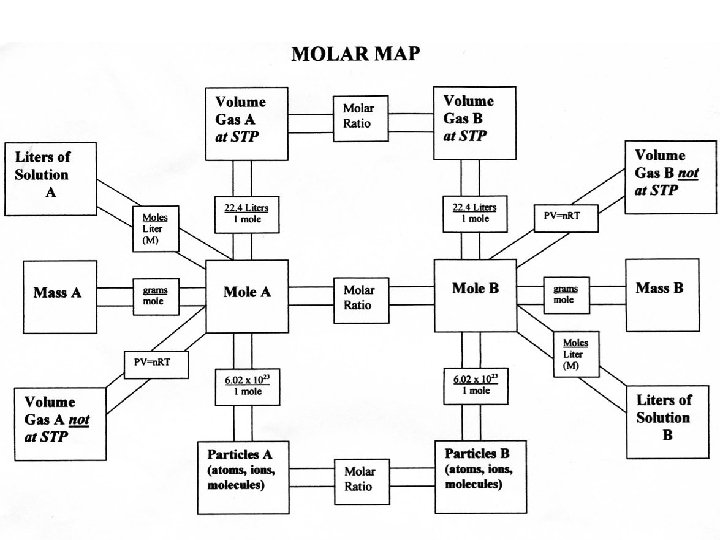
Mole Map
- Slides: 15