Chemistry 1 Notes 2 b Chapter 10 Chemical

Chemistry 1 – Notes #2 b Chapter 10 Chemical Reactions
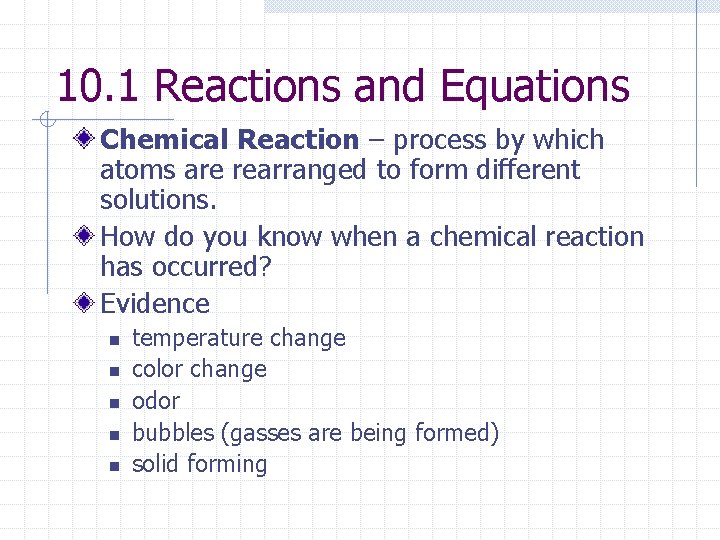
10. 1 Reactions and Equations Chemical Reaction – process by which atoms are rearranged to form different solutions. How do you know when a chemical reaction has occurred? Evidence n n n temperature change color change odor bubbles (gasses are being formed) solid forming
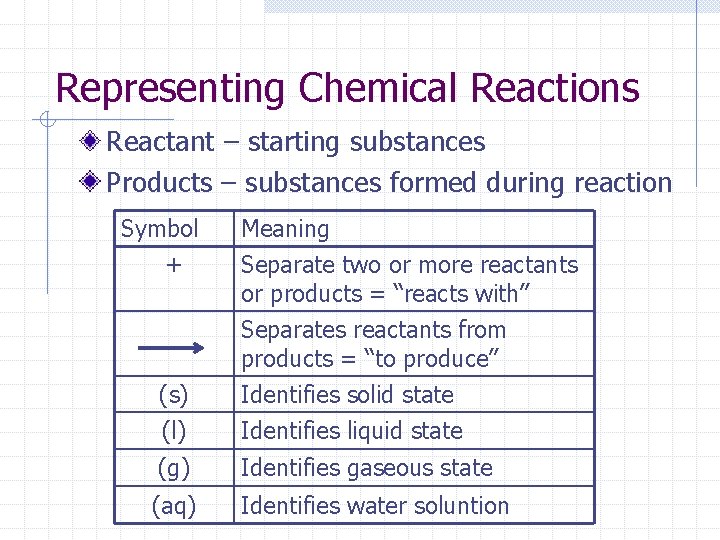
Representing Chemical Reactions Reactant – starting substances Products – substances formed during reaction Symbol + Meaning Separate two or more reactants or products = “reacts with” Separates reactants from products = “to produce” (s) (l) Identifies solid state Identifies liquid state (g) Identifies gaseous state (aq) Identifies water soluntion
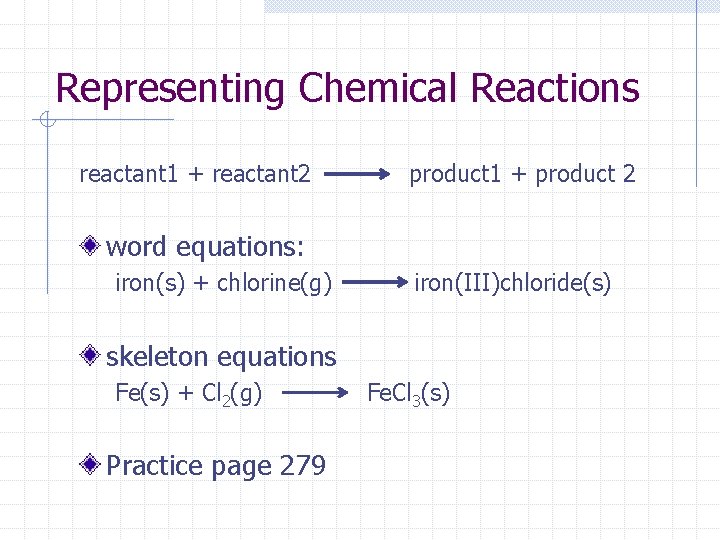
Representing Chemical Reactions reactant 1 + reactant 2 product 1 + product 2 word equations: iron(s) + chlorine(g) iron(III)chloride(s) skeleton equations Fe(s) + Cl 2(g) Practice page 279 Fe. Cl 3(s)
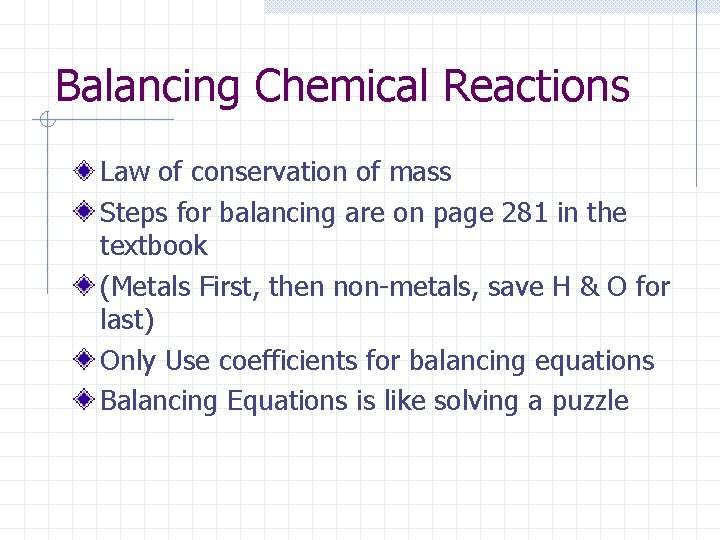
Balancing Chemical Reactions Law of conservation of mass Steps for balancing are on page 281 in the textbook (Metals First, then non-metals, save H & O for last) Only Use coefficients for balancing equations Balancing Equations is like solving a puzzle

10. 2 Classifying Chemical Reactions 5 types of chemical reactions 1. Synthesis 2. Combustion 3. Decomposition 4. Single Replacement 5. Double Replacement some reactions may fit into more than one category
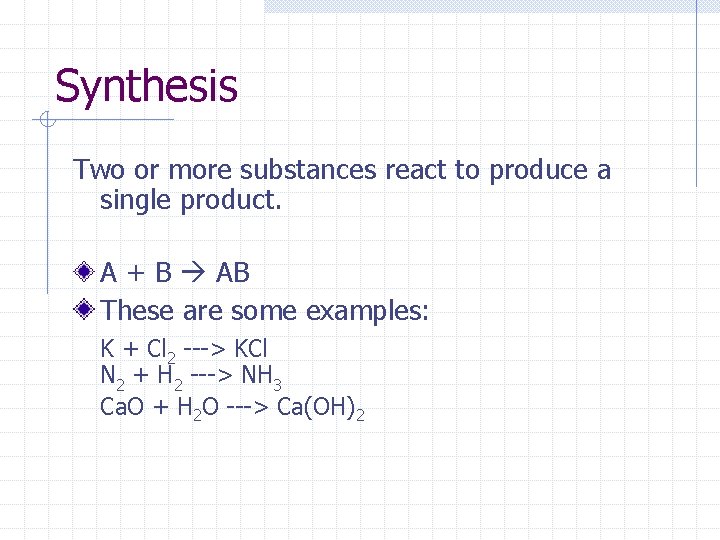
Synthesis Two or more substances react to produce a single product. A + B AB These are some examples: K + Cl 2 ---> KCl N 2 + H 2 ---> NH 3 Ca. O + H 2 O ---> Ca(OH)2
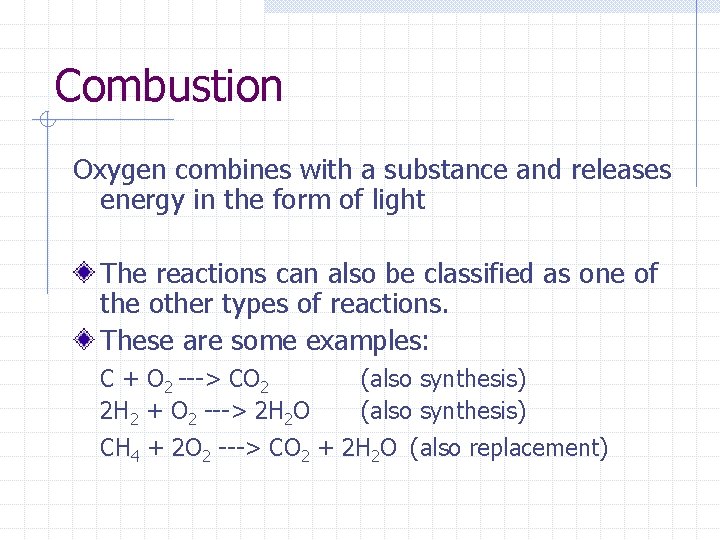
Combustion Oxygen combines with a substance and releases energy in the form of light The reactions can also be classified as one of the other types of reactions. These are some examples: C + O 2 ---> CO 2 2 H 2 + O 2 ---> 2 H 2 O (also synthesis) CH 4 + 2 O 2 ---> CO 2 + 2 H 2 O (also replacement)
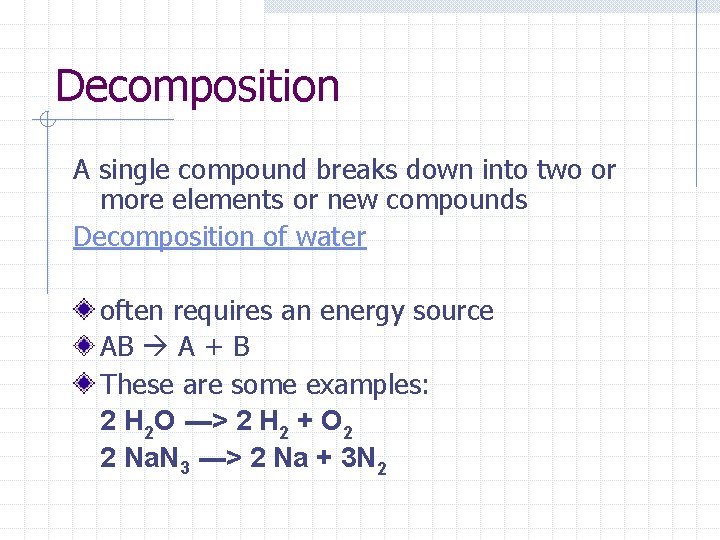
Decomposition A single compound breaks down into two or more elements or new compounds Decomposition of water often requires an energy source AB A + B These are some examples: 2 H 2 O ---> 2 H 2 + O 2 2 Na. N 3 ---> 2 Na + 3 N 2
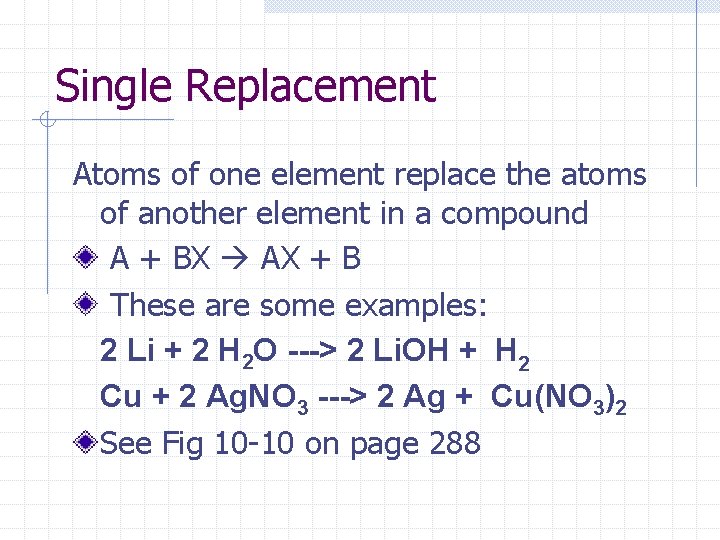
Single Replacement Atoms of one element replace the atoms of another element in a compound A + BX AX + B These are some examples: 2 Li + 2 H 2 O ---> 2 Li. OH + H 2 Cu + 2 Ag. NO 3 ---> 2 Ag + Cu(NO 3)2 See Fig 10 -10 on page 288
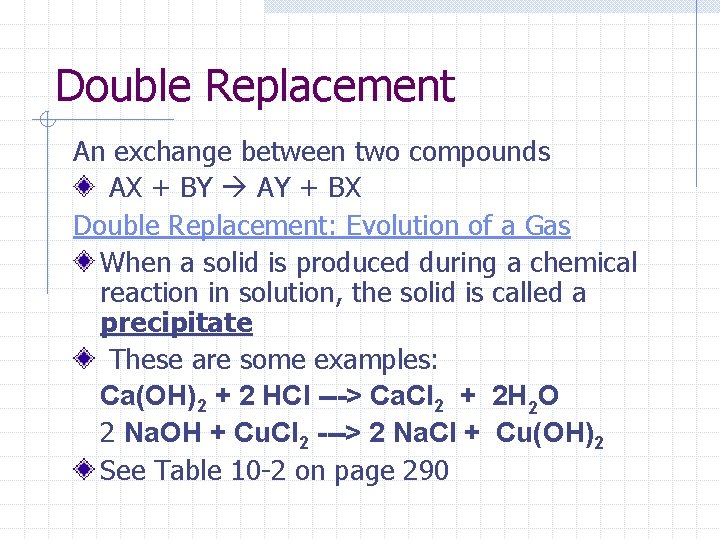
Double Replacement An exchange between two compounds AX + BY AY + BX Double Replacement: Evolution of a Gas When a solid is produced during a chemical reaction in solution, the solid is called a precipitate These are some examples: Ca(OH)2 + 2 HCl ---> Ca. Cl 2 + 2 H 2 O 2 Na. OH + Cu. Cl 2 ---> 2 Na. Cl + Cu(OH)2 See Table 10 -2 on page 290

10. 3 Reactions in Aqueous Solutions Vocabulary to Know Solution – homogeneous mixture Solute – a substance dissolved in a solution Solvent – the substance that dissolves a solute to form a solution Aqueous solution – a solution in which the solvent is water.
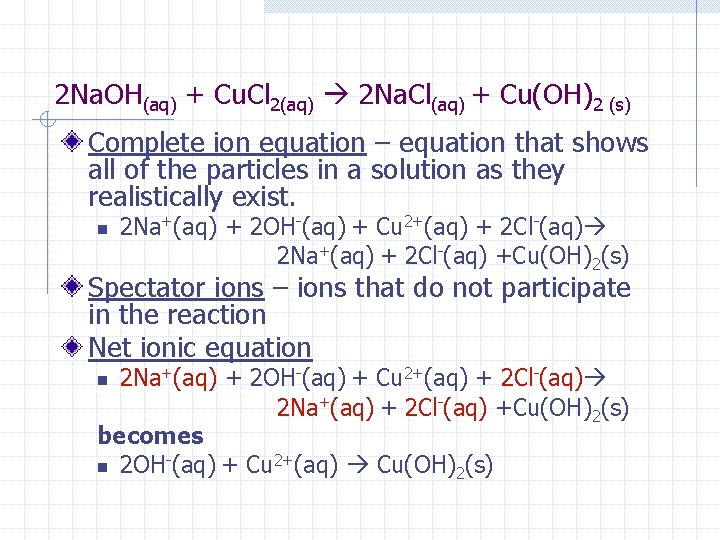
2 Na. OH(aq) + Cu. Cl 2(aq) 2 Na. Cl(aq) + Cu(OH)2 (s) Complete ion equation – equation that shows all of the particles in a solution as they realistically exist. n 2 Na+(aq) + 2 OH-(aq) + Cu 2+(aq) + 2 Cl-(aq) 2 Na+(aq) + 2 Cl-(aq) +Cu(OH)2(s) Spectator ions – ions that do not participate in the reaction Net ionic equation 2 Na+(aq) + 2 OH-(aq) + Cu 2+(aq) + 2 Cl-(aq) 2 Na+(aq) + 2 Cl-(aq) +Cu(OH)2(s) becomes n 2 OH-(aq) + Cu 2+(aq) Cu(OH)2(s) n
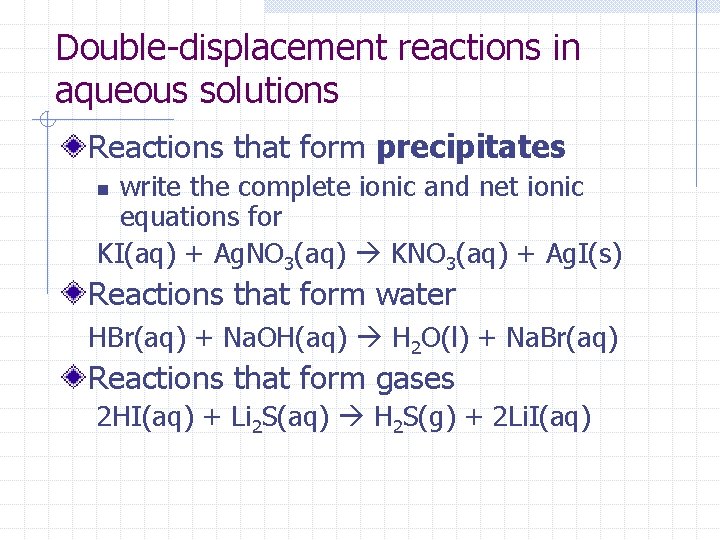
Double-displacement reactions in aqueous solutions Reactions that form precipitates write the complete ionic and net ionic equations for KI(aq) + Ag. NO 3(aq) KNO 3(aq) + Ag. I(s) n Reactions that form water HBr(aq) + Na. OH(aq) H 2 O(l) + Na. Br(aq) Reactions that form gases 2 HI(aq) + Li 2 S(aq) H 2 S(g) + 2 Li. I(aq)
- Slides: 14