CHEMICAL VS PHYSICAL PROPERTIES SO FAR We have













- Slides: 15

CHEMICAL VS. PHYSICAL PROPERTIES

SO FAR. . . We have defined chemistry: The study of matter and its reactions What is matter? What is a reaction?

FORMAL DEFINITION The formal definition of matter is: Anything that has mass and takes up space We are going to go over some ways we describe matter

PHYSICAL PROPERTIES Physical property: a characteristic of matter that can be observed without changing the composition of matter Density Color Odor Hardness Melting point Boiling point

CHEMICAL PROPERTIES Chemical property: ability to combine with or change into one or more other substances How does it react with other substances

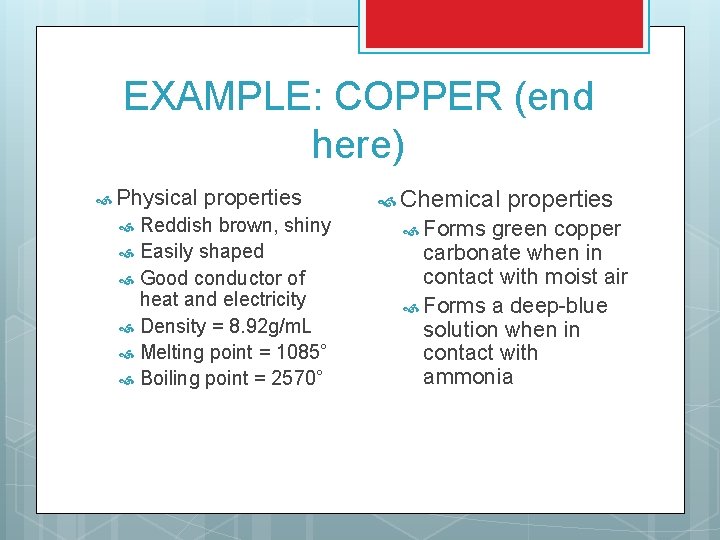
EXAMPLE: COPPER (end here) Physical properties Reddish brown, shiny Easily shaped Good conductor of heat and electricity Density = 8. 92 g/m. L Melting point = 1085° Boiling point = 2570° Chemical Forms properties green copper carbonate when in contact with moist air Forms a deep-blue solution when in contact with ammonia

PHYSICAL CHANGE Physical change: a change in a substance that does not change the composition of the substance Boiling Melting Breaking/bending

CHEMICAL CHANGE Chemical change: a change that produces a new substance Explosion Rust Corrosion Burn

PHASE CHANGES We’ve already discussed one type of physical property – DENSITY We are now going to focus on an important type of physical change PHASE CHANGES

PHASES OF MATTER Phase: form of matter. Solid: phase of matter w/ a definite shape and volume. Liquid: phase of matter w/ definite volume, but no shape. Gas: phase of matter that has no definite shape or volume

HOW DOES MATTER CHANGE PHASES? Evaporation: change from a liquid to a gas. Condensation: change from a gas to a liquid. Freezing: change from a liquid to a solid. Melting: change from a solid to a liquid.

WHAT DO THE PARTICLES LOOK LIKE? For each state of matter, the particles that make up the substance behave differently. In your notes, you will draw how the molecules are arranged for the following: Solid Liquid Gas

WHAT HAPPENS IN A PHASE CHANGE Next, in your notes, I want you to write a paragraph about how the molecules behave when you change from one state of matter to another: Solid Liquid Gas

PHASE CHANGE DIAGRAM We are going to create a phase change diagram showing how temperature behaves when we change states of matter

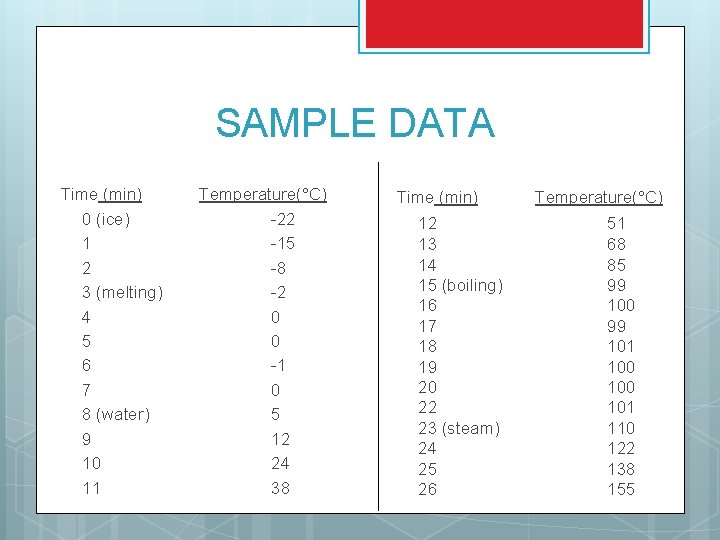
SAMPLE DATA Time (min) 0 (ice) 1 2 3 (melting) 4 5 6 7 8 (water) 9 10 11 Temperature(°C) -22 -15 -8 -2 0 0 -1 0 5 12 24 38 Time (min) 12 13 14 15 (boiling) 16 17 18 19 20 22 23 (steam) 24 25 26 Temperature(°C) 51 68 85 99 100 99 101 100 101 110 122 138 155