Chemical vs Physical Changes Physical Change qthe substances

Chemical vs. Physical Changes

Physical Change qthe substances are not altered chemically, but are merely changed to another phase (i. e. gas, liquid, solid) or separated or combined. • No change occurs in the identity of the substance
Chemical change qthe substances are altered chemically and display different physical and chemical properties after the change. q. Atoms in the reactants are rearranged to form one or more different substances q. Old bonds are broken; new bonds form
Indicators/Signs of a Chemical Change (these are not fool proof) n Chemists Get Practice Trying Labs – – Color change Gas/bubbles Precipitate, ppt (a solid forms) Temperature change n Exothermic (hot) or endothermic (cold) – Light given off v. Typically, more than one indicator will be seen.
Learning Check Classify each of the following as a 1) physical change or 2) chemical change A. ____ a burning candle B. ____ melting ice C. ____ toasting a marshmallow D. ____ cutting a pizza E. ____ Tarnished silver

Solution Classify each of the following as a 1) physical change or 2) chemical change A. __2__ a burning candle B. __1_ melting ice C. __2__ toasting a marshmallow D. __1__ cutting a pizza E. __2__ tarnished silver

Chemical Reaction A process in which at least one new substance is produced as a result of chemical change.

In a chemical reaction The way atoms are joined is changed n Atoms aren’t created or destroyed. n Can be described several ways: n – In a sentence n Copper reacts with chlorine to form copper (II) chloride. – In a word equation n Copper + chlorine ® copper (II) chloride – Formulas n Cu+ Cl 2 Cu. Cl 2
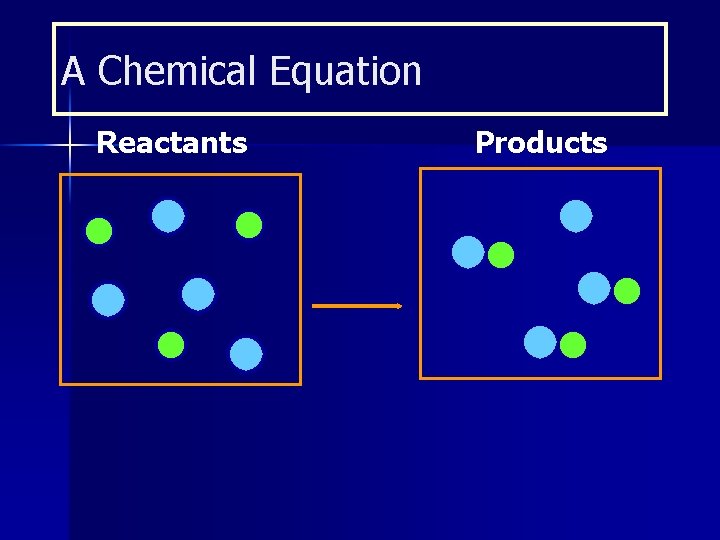
A Chemical Equation Reactants Products
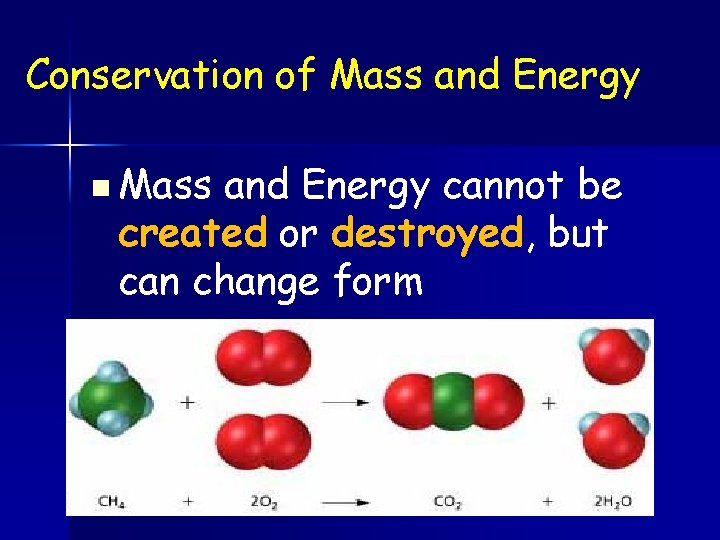
Conservation of Mass and Energy n Mass and Energy cannot be created or destroyed, but can change form
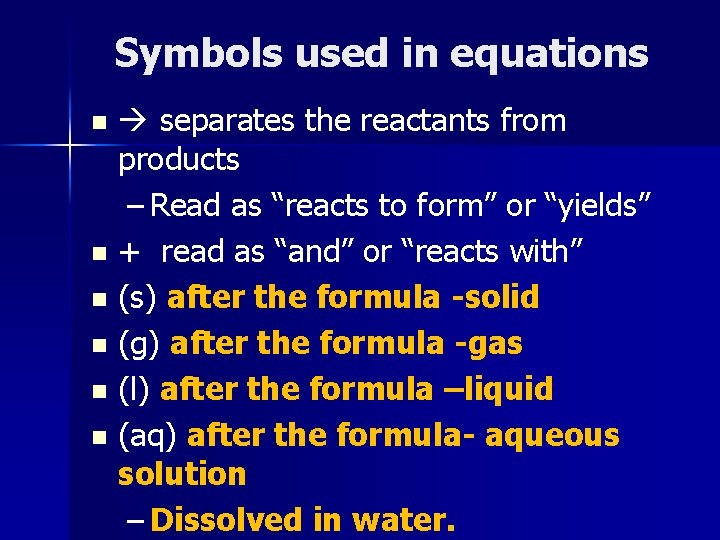
Symbols used in equations separates the reactants from products – Read as “reacts to form” or “yields” n + read as “and” or “reacts with” n (s) after the formula -solid n (g) after the formula -gas n (l) after the formula –liquid n (aq) after the formula- aqueous solution – Dissolved in water. n
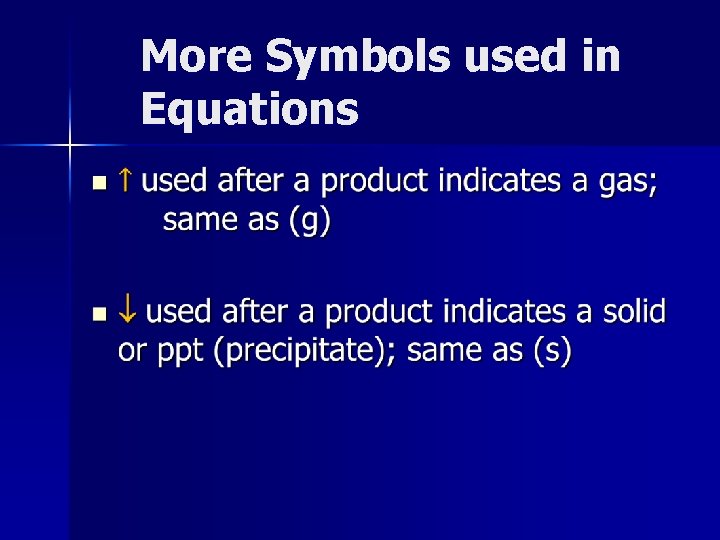
More Symbols used in Equations n
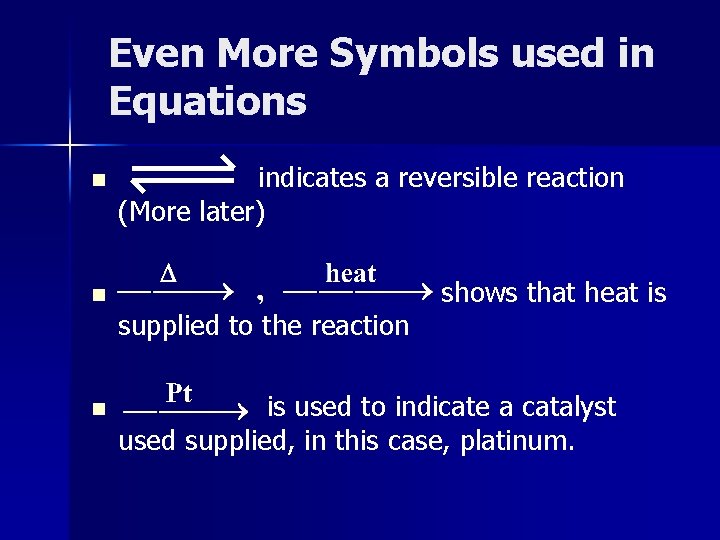
Even More Symbols used in Equations n indicates a reversible reaction (More later) n shows that heat is supplied to the reaction n is used to indicate a catalyst used supplied, in this case, platinum.
What is a catalyst? A substance that speeds up a reaction without being changed by the reaction. n Enzymes are biological or protein catalysts. n

Diatomic elements There are 8 elements that never want to be alone. n If they are not with another element, they form diatomic molecules. n H 2 , N 2 , O 2 , F 2 , Cl 2 , Br 2 , I 2 , and At 2 n Brother HON CLIF n 1 + 7 pattern on the periodic table n


Sulfur & Phosphorus Sulfur, when alone, exists as S 8 Phosphorus, when alone, exists as P 4
- Slides: 17