Chemical Synthesis Lesson 2 Learning objective To explain

Chemical Synthesis Lesson 2

Learning objective: To explain the reactions involving acids. • Must: Describe the p. H scale. Grade D • Must: Describe what happens during a neutralisation reaction. Grade C • Should: Predict the name of products formed during acid reactions. Grade B • Could: Write balanced symbol equations for the acid reactions. Grade A • Keywords: p. H scale, salt, acid, word equation and balanced equation.

Let’s make a rainbow. • Key skill used. • That you can read and understand texts and take the appropriate action. • As a team worker you can collaborate with others to work towards common goals.
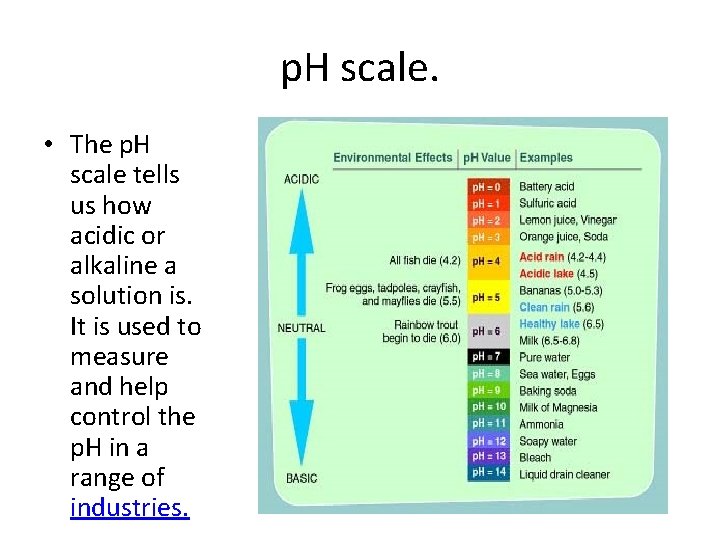
p. H scale. • The p. H scale tells us how acidic or alkaline a solution is. It is used to measure and help control the p. H in a range of industries.

p. H scale 1. Indicators can be used to find out whether a solution is acid, alkaline or neutral 2. Universal Indicator can be used to find the p. H of a solution • Acid – red, p. H less than 7 • Neutral – green, p. H = 7 • Alkali – blue, p. H greater than 7
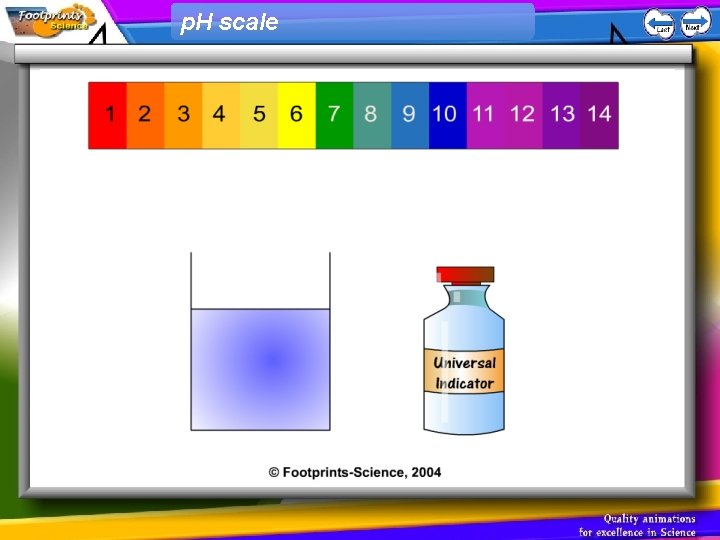
p. H scale

p. H scale

Important acids. • Hydrochloric acid – HCl(aq) • Nitric acid– HNO 3(aq) • Sulfuric acid – H 2 SO 4(aq) • Make sure you learn and remember them! • What does the (aq) mean?
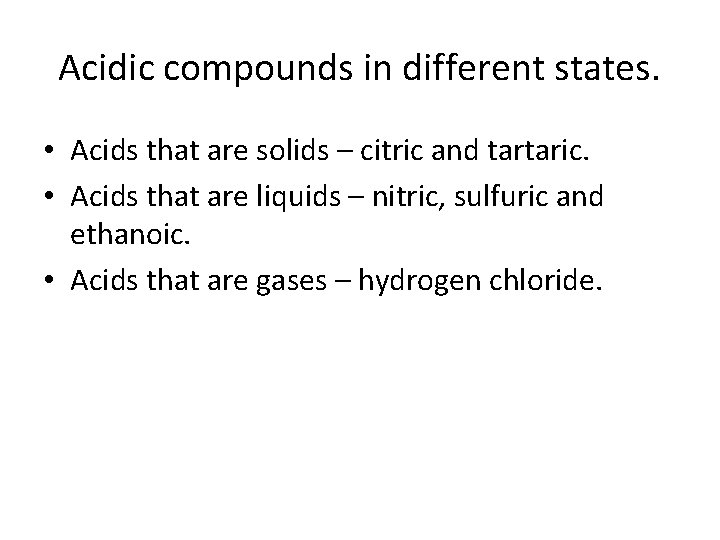
Acidic compounds in different states. • Acids that are solids – citric and tartaric. • Acids that are liquids – nitric, sulfuric and ethanoic. • Acids that are gases – hydrogen chloride.

Formula of some alkalis. • Sodium hydroxide – Na. OH • Potassium hydroxide – KOH • Magnesium hydroxide – Mg(OH)2 • Learn and remember these!

What is an acid? • All acids contain hydrogen atoms. • When acids dissolve in water they produce hydrogen ions, H+. • HCl(g) H+(aq) + Cl-(aq) • That is why acids only behave like acids when they are dissolved in water.

What about alkalis? • The same can be applied to alkalis. • This time it is the presence of the OH- ion that makes a substance an alkali. • Strong alkalis like Na. OH sodium hydroxide split up completely. • Na. OH(aq) Na+(aq) + OH-(aq)

What happens to the ions during neutralisation.
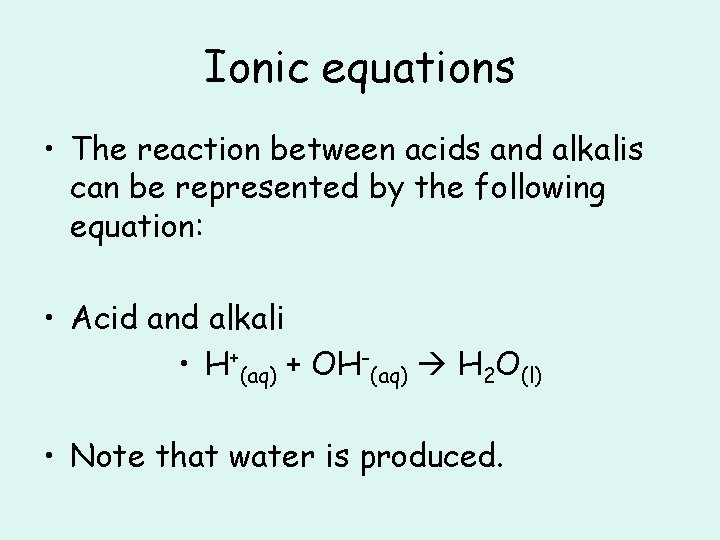
Ionic equations • The reaction between acids and alkalis can be represented by the following equation: • Acid and alkali • H+(aq) + OH-(aq) H 2 O(l) • Note that water is produced.

Learning objective: To explain the reactions involving acids. • Must: Describe the p. H scale. • Must: Describe what happens during a neutralisation reaction. • Should: Predict the name of products formed during acid reactions. • Could: Write balanced symbol equations for the acid reactions. • Keywords: p. H scale, salt, acid, word equation and balanced equation.
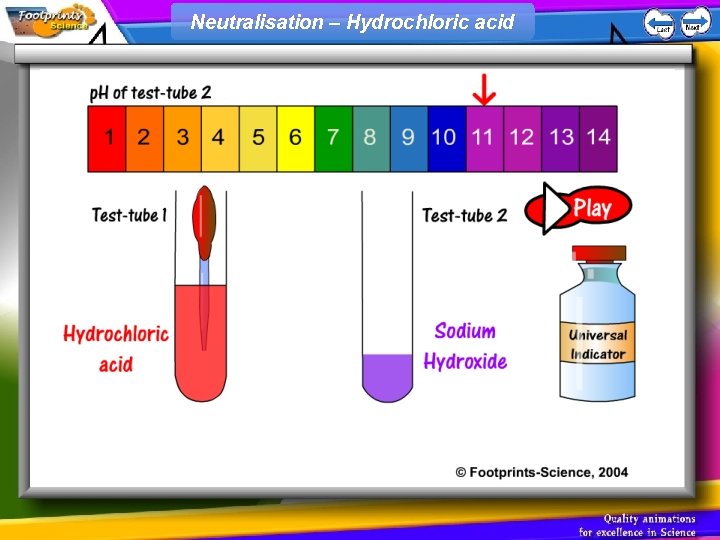
Neutralisation – Hydrochloric acid
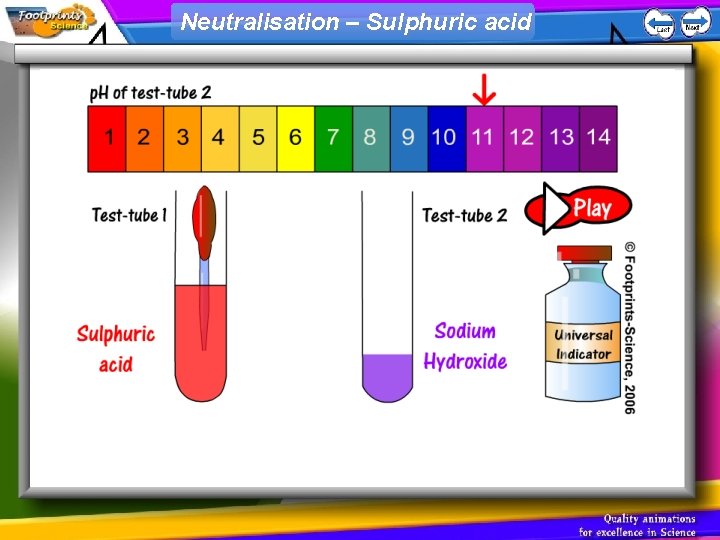
Neutralisation – Sulphuric acid
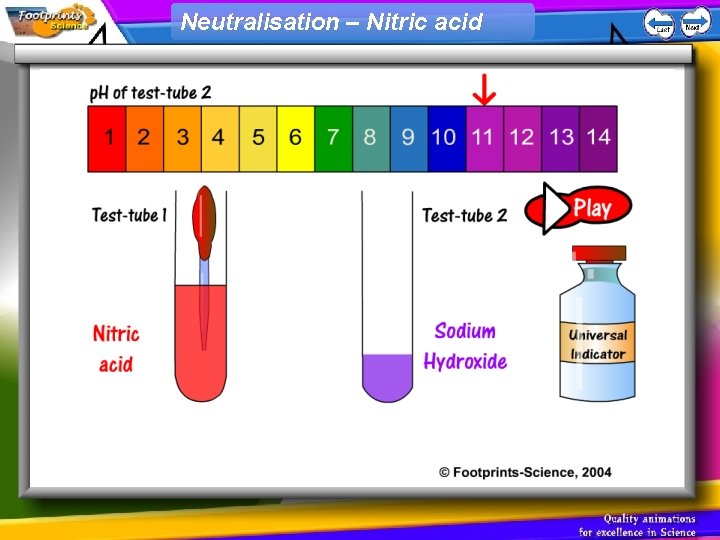
Neutralisation – Nitric acid

Discover - Monitoring Neutralisation. • Follow the instructions for AC 6. 13. • Add 5 cm 3 of HCl up to 35 cm 3 and then 1 cm 3 up to 45 cm 3 • Record your results and plot the graph. • Make sure you wear goggles. • Key skills • Read and understand texts and take appropriate action. • Use appropriate mathematical procedures. • Examine patterns and relationships. • Team worker – collaborate with others to work towards common goals.
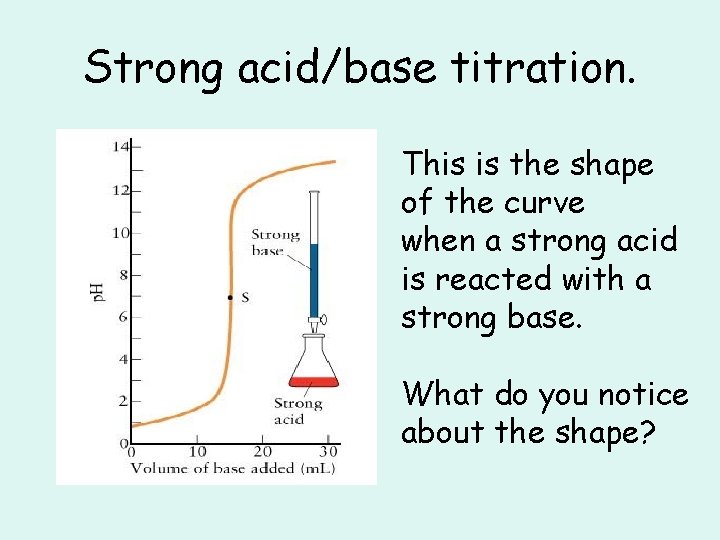
Strong acid/base titration. This is the shape of the curve when a strong acid is reacted with a strong base. What do you notice about the shape?

Testing understanding. • Click here for more examples of neutralisation reactions. • Click here for more information and tests about acids and alkalis.

Learning objective: To explain the reactions involving acids. • Must: Describe the p. H scale. • Must: Describe what happens during a neutralisation reaction. • Should: Predict the name of products formed during acid reactions. • Could: Write balanced symbol equations for the acid reactions. • Keywords: p. H scale, salt, acid, word equation and balanced equation.

Discover - Investigating acids. • Follow the instructions for AC 6. 3. • Record your results and write word equations for each reaction. • Make sure you are wearing goggles. • Key skills. • Read and understand texts and take appropriate action. • Team worker – collaborate with others to work towards common goals.

Naming Salts 1. Acids react with alkaline solutions to form a salt and water • Hydrochloric acid produces chlorides • Nitric acid produces nitrates • Sulphuric acid produces sulphates
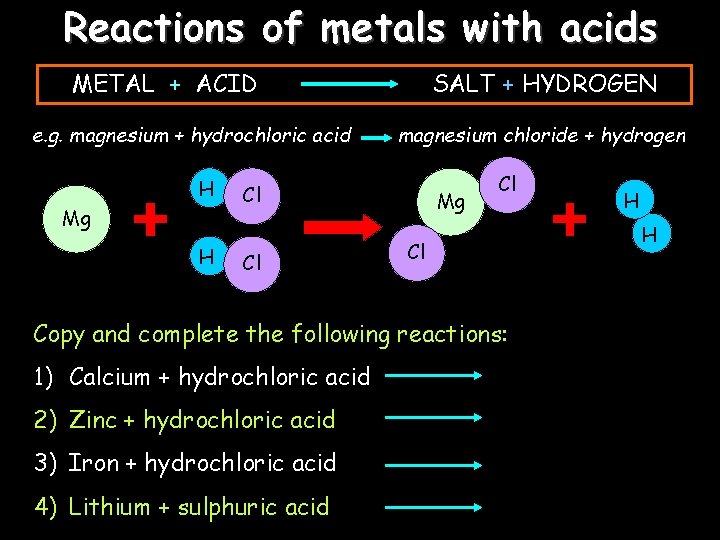
Reactions of metals with acids METAL + ACID e. g. magnesium + hydrochloric acid H Mg H SALT + HYDROGEN magnesium chloride + hydrogen Cl Cl Mg Cl Cl Copy and complete the following reactions: 1) Calcium + hydrochloric acid 2) Zinc + hydrochloric acid 3) Iron + hydrochloric acid 4) Lithium + sulphuric acid H H
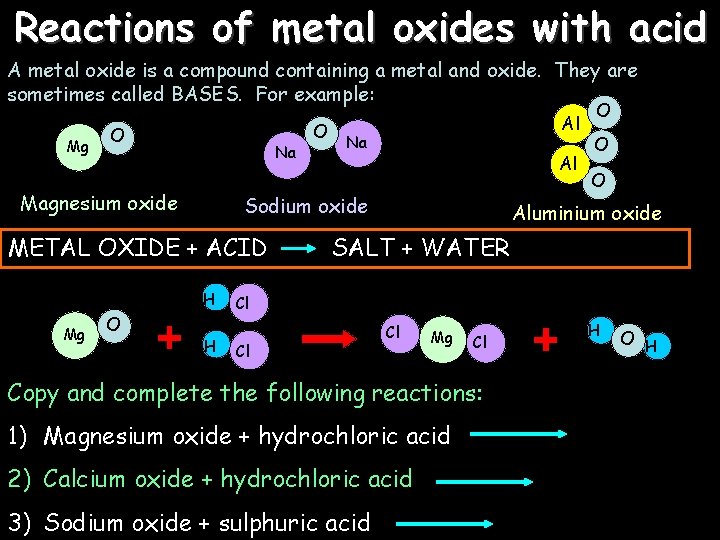
Reactions of metal oxides with acid A metal oxide is a compound containing a metal and oxide. They are sometimes called BASES. For example: Mg O Na Magnesium oxide H O Na Al Sodium oxide METAL OXIDE + ACID Mg O Al H O O O Aluminium oxide SALT + WATER Cl Cl Cl Mg Cl Copy and complete the following reactions: 1) Magnesium oxide + hydrochloric acid 2) Calcium oxide + hydrochloric acid 3) Sodium oxide + sulphuric acid H O H
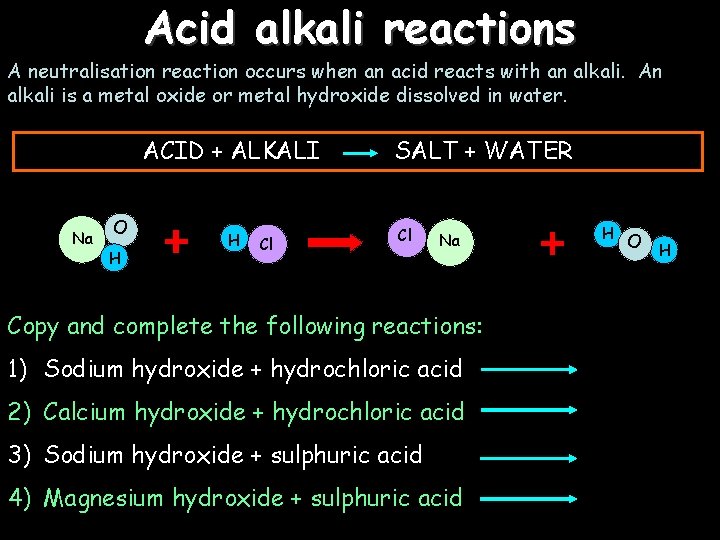
Acid alkali reactions A neutralisation reaction occurs when an acid reacts with an alkali. An alkali is a metal oxide or metal hydroxide dissolved in water. ACID + ALKALI Na O H H Cl SALT + WATER Cl Na Copy and complete the following reactions: 1) Sodium hydroxide + hydrochloric acid 2) Calcium hydroxide + hydrochloric acid 3) Sodium hydroxide + sulphuric acid 4) Magnesium hydroxide + sulphuric acid H O H
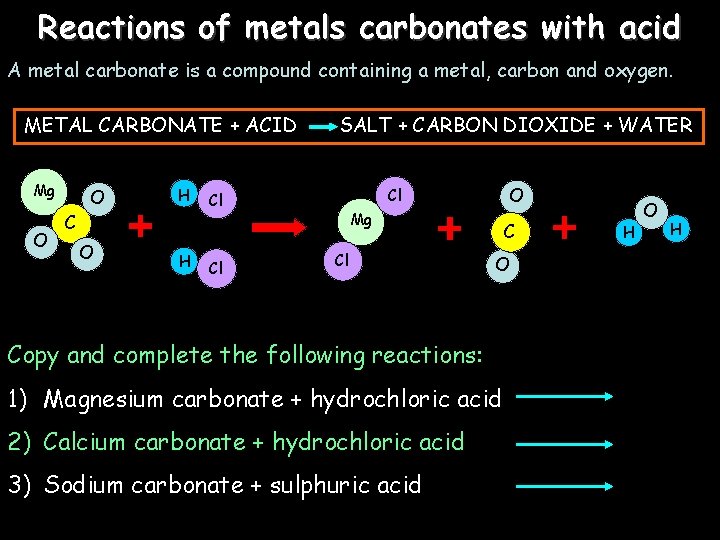
Reactions of metals carbonates with acid A metal carbonate is a compound containing a metal, carbon and oxygen. METAL CARBONATE + ACID Mg O O H C O H SALT + CARBON DIOXIDE + WATER Cl O Cl Cl Mg Cl C O Copy and complete the following reactions: 1) Magnesium carbonate + hydrochloric acid 2) Calcium carbonate + hydrochloric acid 3) Sodium carbonate + sulphuric acid H O H

Testing understanding. • Click here for more word and symbol equations for reactions involving acids. • Click here for revision notes and test on reactions involving acids.
- Slides: 29