Chemical Symbols Formulas Equations Chemical Symbols A symbol
Chemical Symbols, Formulas & Equations
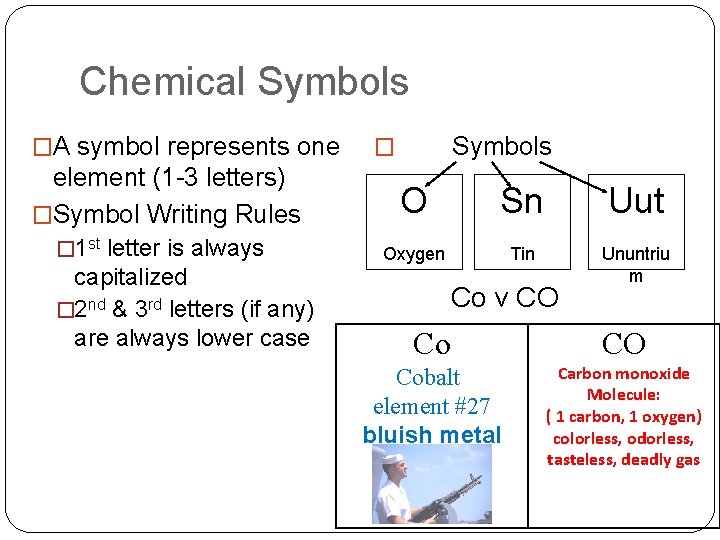
Chemical Symbols �A symbol represents one element (1 -3 letters) �Symbol Writing Rules � 1 st letter is always capitalized � 2 nd & 3 rd letters (if any) are always lower case Symbols � O Sn Uut Oxygen Tin Ununtriu m Co v CO Cobalt element #27 bluish metal Carbon monoxide Molecule: ( 1 carbon, 1 oxygen) colorless, odorless, tasteless, deadly gas

IUPAC Naming New Elements � New elements are being synthesized (made) in the lab � Names for the element has to wait until the IUPAC naming committee meets � A temporary name based on the atomic number IN LATIN is given to the new element � Time to learn some Latin • 1 – un • 4 – • 7 – sept 0 -nil • 2 – bi quad • 8 – oct • 3 – tri • 5 – pent • 9 – enn • 6 elements – hex � Rules for naming Break up the atomic number into digits (#118 becomes 1, 1, 8) 2. Replace the digits with the Latin number (1, 1, 8 becomes un un oct 3. Put the Latin numbers together and add “–ium” ending – ununoctium 1.
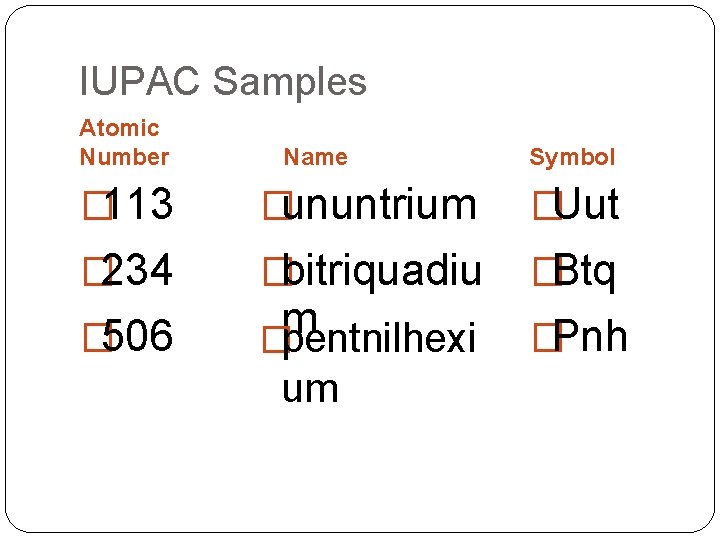
IUPAC Samples Atomic Number Name Symbol � 113 �ununtrium �Uut � 234 �bitriquadiu �Btq � 506 m �pentnilhexi um �Pnh
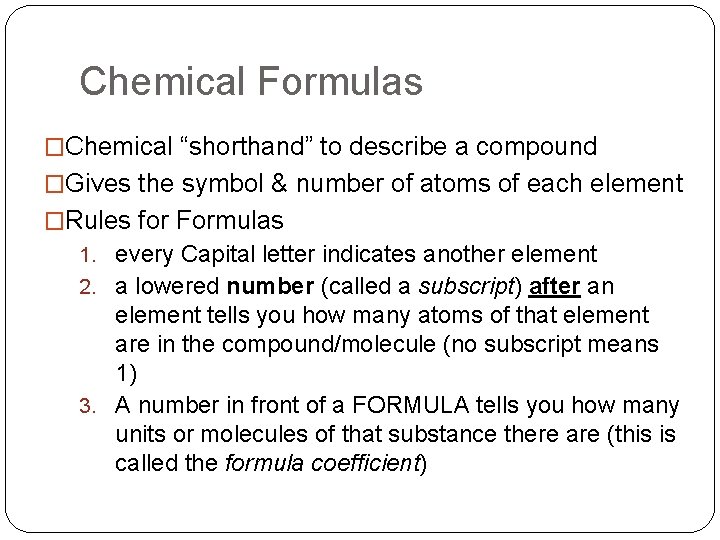
Chemical Formulas �Chemical “shorthand” to describe a compound �Gives the symbol & number of atoms of each element �Rules for Formulas 1. every Capital letter indicates another element 2. a lowered number (called a subscript) after an element tells you how many atoms of that element are in the compound/molecule (no subscript means 1) 3. A number in front of a FORMULA tells you how many units or molecules of that substance there are (this is called the formula coefficient)
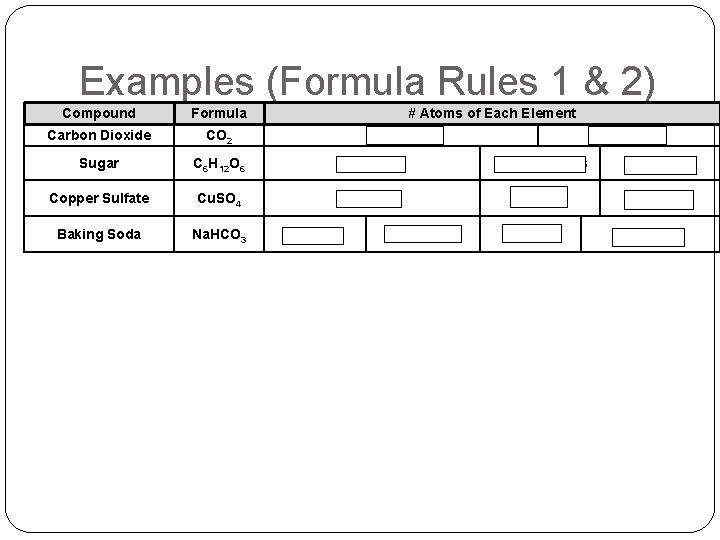
Examples (Formula Rules 1 & 2) Compound Formula # Atoms of Each Element Carbon Dioxide CO 2 Sugar C 6 H 12 O 6 6 -Carbons 12 - Hydrogens 6 -Oxygens Copper Sulfate Cu. SO 4 1 -Copper 1 -Sulfur 4 -Oxygens Baking Soda Na. HCO 3 1 -Carbon 1 -Sodium 1 -Hydrogen 2 - Oxygens 1 -Carbon 3 -Oxygens
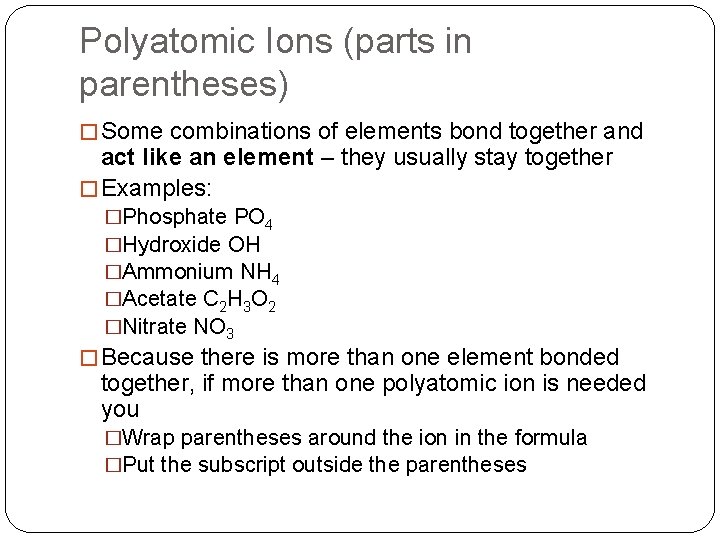
Polyatomic Ions (parts in parentheses) � Some combinations of elements bond together and act like an element – they usually stay together � Examples: �Phosphate PO 4 �Hydroxide OH �Ammonium NH 4 �Acetate C 2 H 3 O 2 �Nitrate NO 3 � Because there is more than one element bonded together, if more than one polyatomic ion is needed you �Wrap parentheses around the ion in the formula �Put the subscript outside the parentheses
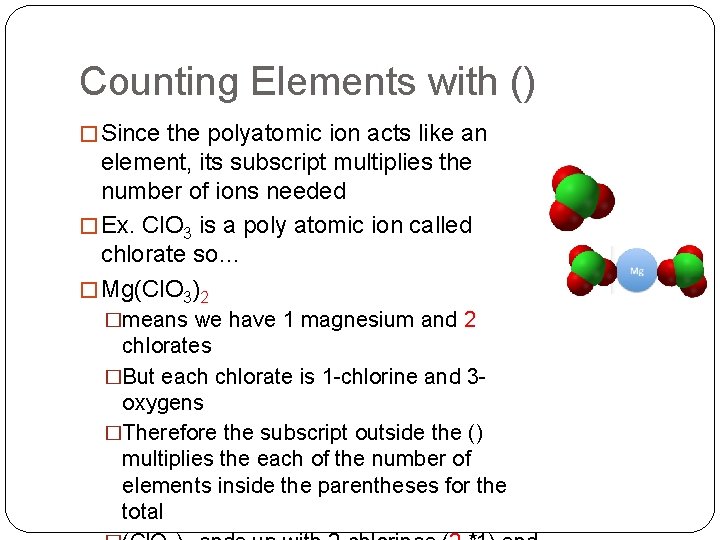
Counting Elements with () � Since the polyatomic ion acts like an element, its subscript multiplies the number of ions needed � Ex. Cl. O 3 is a poly atomic ion called chlorate so… � Mg(Cl. O 3)2 �means we have 1 magnesium and 2 chlorates �But each chlorate is 1 -chlorine and 3 oxygens �Therefore the subscript outside the () multiplies the each of the number of elements inside the parentheses for the total
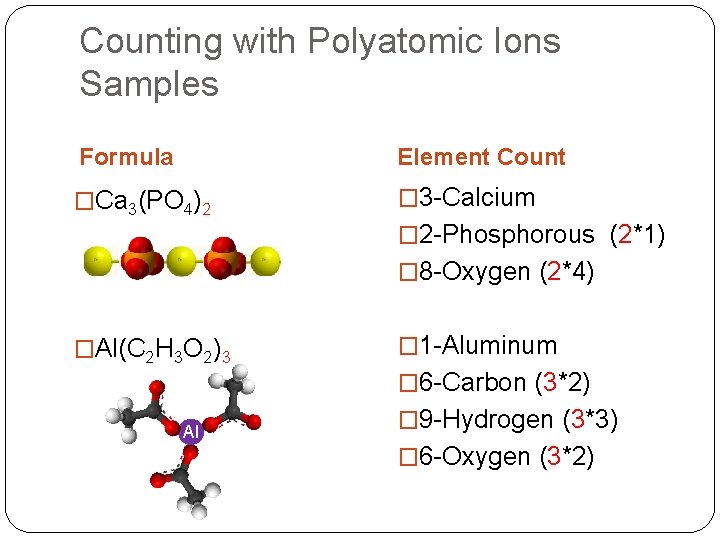
Counting with Polyatomic Ions Samples Formula Element Count �Ca 3(PO 4)2 � 3 -Calcium � 2 -Phosphorous (2*1) � 8 -Oxygen (2*4) �Al(C 2 H 3 O 2)3 � 1 -Aluminum � 6 -Carbon (3*2) Al � 9 -Hydrogen (3*3) � 6 -Oxygen (3*2)
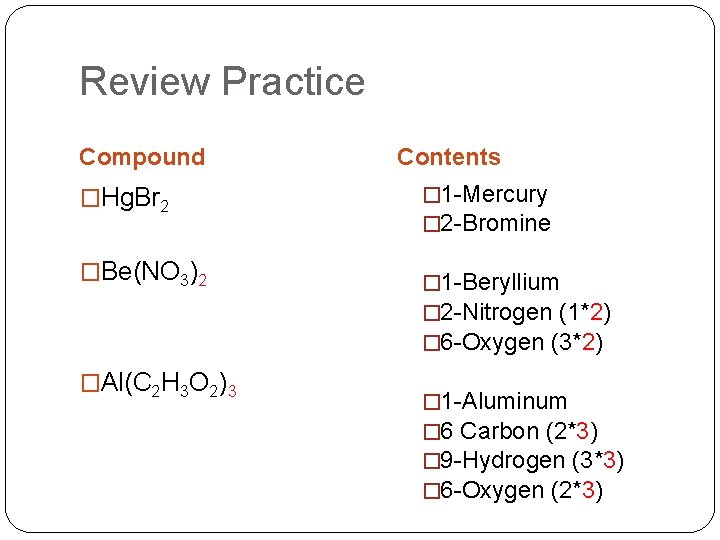
Review Practice Compound �Hg. Br 2 �Be(NO 3)2 �Al(C 2 H 3 O 2)3 Contents � 1 -Mercury � 2 -Bromine � 1 -Beryllium � 2 -Nitrogen (1*2) � 6 -Oxygen (3*2) � 1 -Aluminum � 6 Carbon (2*3) � 9 -Hydrogen (3*3) � 6 -Oxygen (2*3)
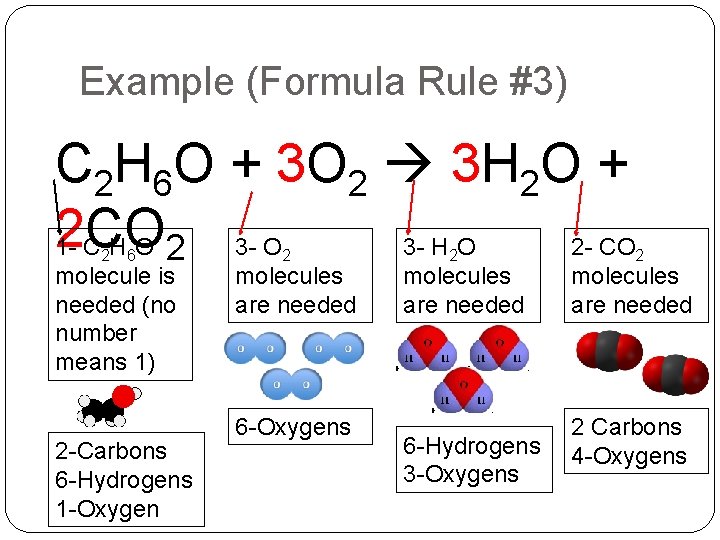
Example (Formula Rule #3) C 2 H 6 O + 3 O 2 3 H 2 O + 2 CO 1 CHO 2 3 - O 3 - H O 2 - CO 2 6 molecule is needed (no number means 1) 2 -Carbons 6 -Hydrogens 1 -Oxygen 2 molecules are needed 6 -Oxygens 2 molecules are needed 6 -Hydrogens 3 -Oxygens 2 molecules are needed 2 Carbons 4 -Oxygens
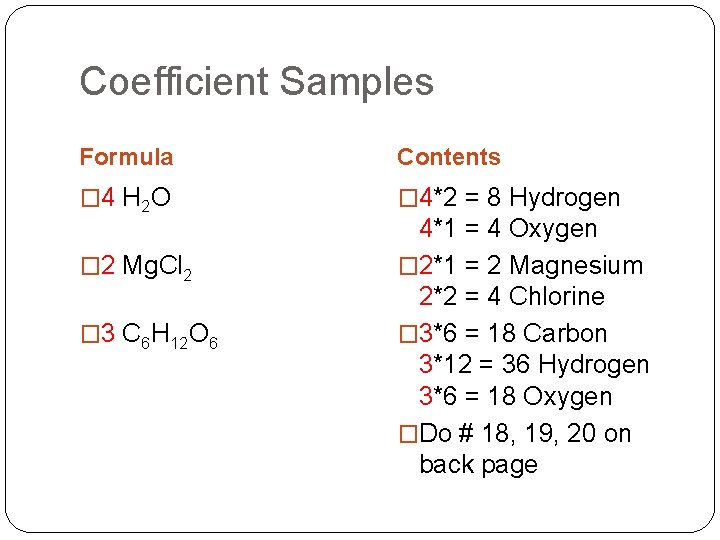
Coefficient Samples Formula Contents � 4 H 2 O � 4*2 = 8 Hydrogen � 2 Mg. Cl 2 � 3 C 6 H 12 O 6 4*1 = 4 Oxygen � 2*1 = 2 Magnesium 2*2 = 4 Chlorine � 3*6 = 18 Carbon 3*12 = 36 Hydrogen 3*6 = 18 Oxygen �Do # 18, 19, 20 on back page
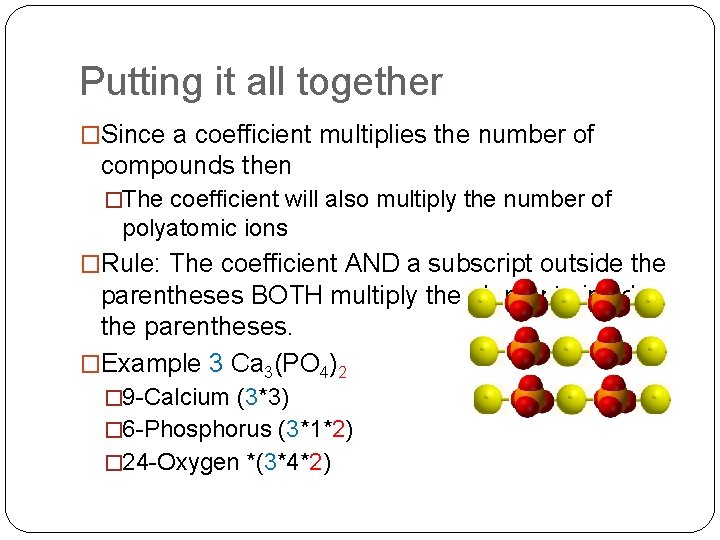
Putting it all together �Since a coefficient multiplies the number of compounds then �The coefficient will also multiply the number of polyatomic ions �Rule: The coefficient AND a subscript outside the parentheses BOTH multiply the elements inside the parentheses. �Example 3 Ca 3(PO 4)2 � 9 -Calcium (3*3) � 6 -Phosphorus (3*1*2) � 24 -Oxygen *(3*4*2)
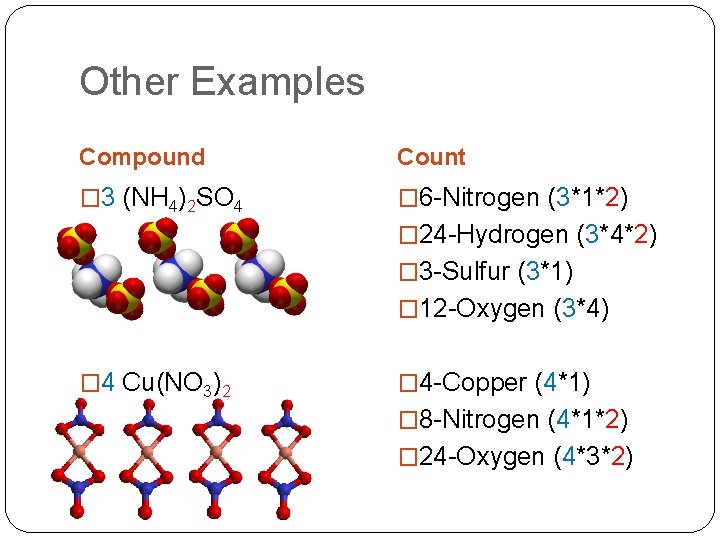
Other Examples Compound Count � 3 (NH 4)2 SO 4 � 6 -Nitrogen (3*1*2) � 24 -Hydrogen (3*4*2) � 3 -Sulfur (3*1) � 12 -Oxygen (3*4) � 4 Cu(NO 3)2 � 4 -Copper (4*1) � 8 -Nitrogen (4*1*2) � 24 -Oxygen (4*3*2)
- Slides: 14