Chemical Symbols and formulas What do we need

Chemical Symbols and formulas What do we need to know in order to write formulas? What do we need to know in order to correctly name compounds?
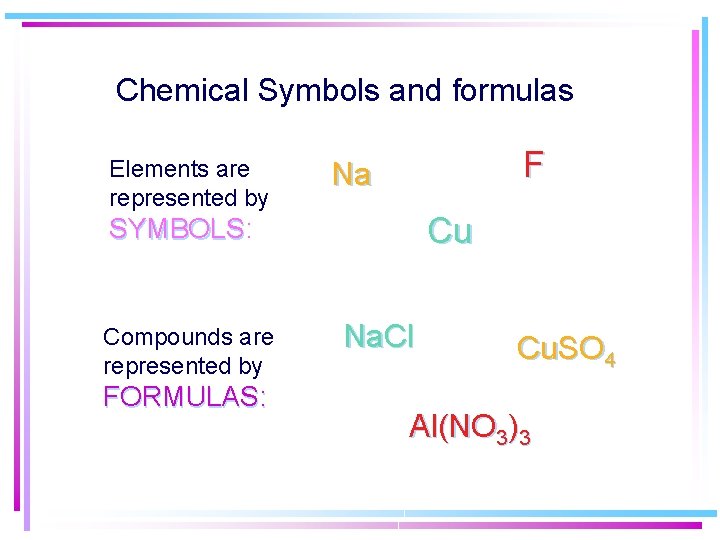
Chemical Symbols and formulas Elements are represented by F Na Cu SYMBOLS: SYMBOLS Compounds are represented by FORMULAS: Na. Cl Cu. SO 4 Al(NO 3)3
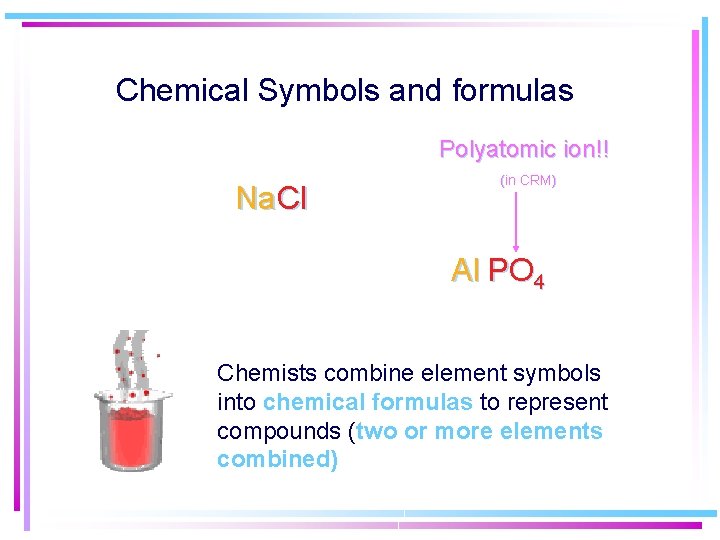
Chemical Symbols and formulas Polyatomic ion!! Na Cl (in CRM) Al PO 4 Chemists combine element symbols into chemical formulas to represent compounds (two or more elements combined)
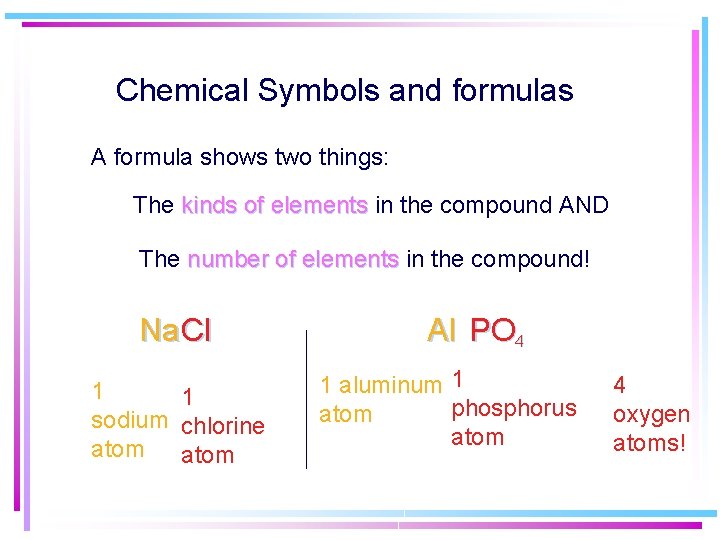
Chemical Symbols and formulas A formula shows two things: The kinds of elements in the compound AND The number of elements in the compound! Na Cl 1 1 sodium chlorine atom Al PO 4 1 aluminum 1 phosphorus atom 4 oxygen atoms!
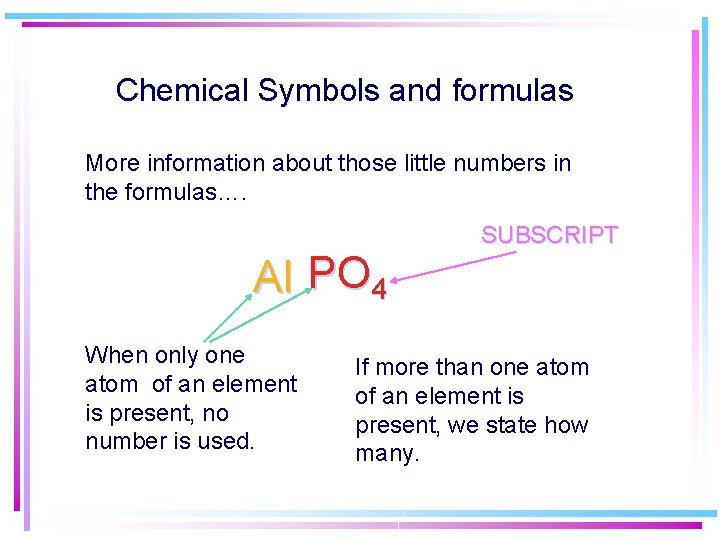
Chemical Symbols and formulas More information about those little numbers in the formulas…. Al PO 4 When only one atom of an element is present, no number is used. SUBSCRIPT If more than one atom of an element is present, we state how many.
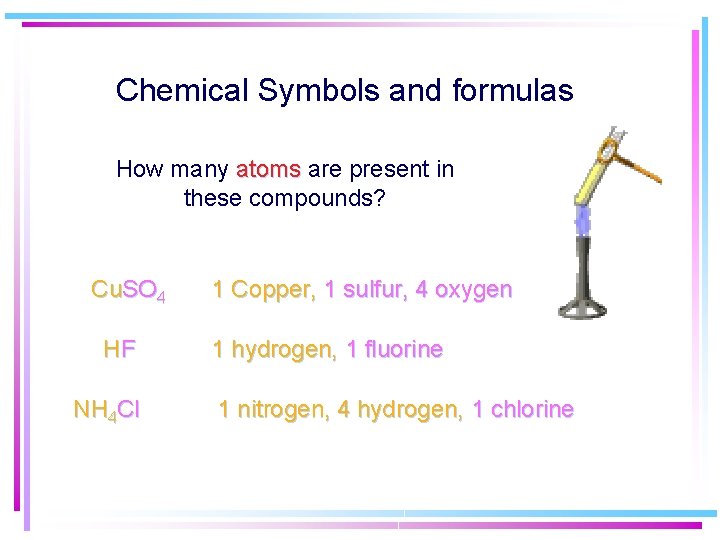
Chemical Symbols and formulas How many atoms are present in these compounds? Cu. SO 4 HF NH 4 Cl 1 Copper, 1 sulfur, 4 oxygen 1 hydrogen, 1 fluorine 1 nitrogen, 4 hydrogen, 1 chlorine
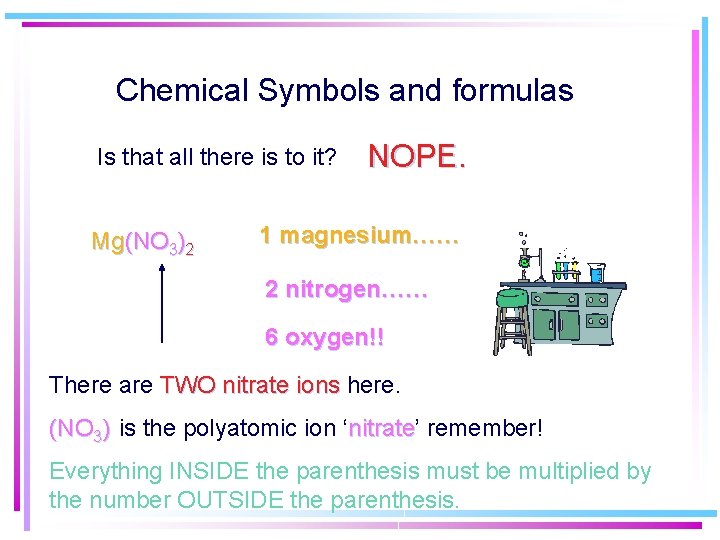
Chemical Symbols and formulas Is that all there is to it? Mg(NO 3)2 NOPE. 1 magnesium…… 2 nitrogen…… 6 oxygen!! There are TWO nitrate ions here. (NO 3) is the polyatomic ion ‘nitrate’ nitrate remember! Everything INSIDE the parenthesis must be multiplied by the number OUTSIDE the parenthesis.
Chemical Symbols and formulas In order to write formulas and correctly name compounds we need to know something about Electrons And the Periodic table
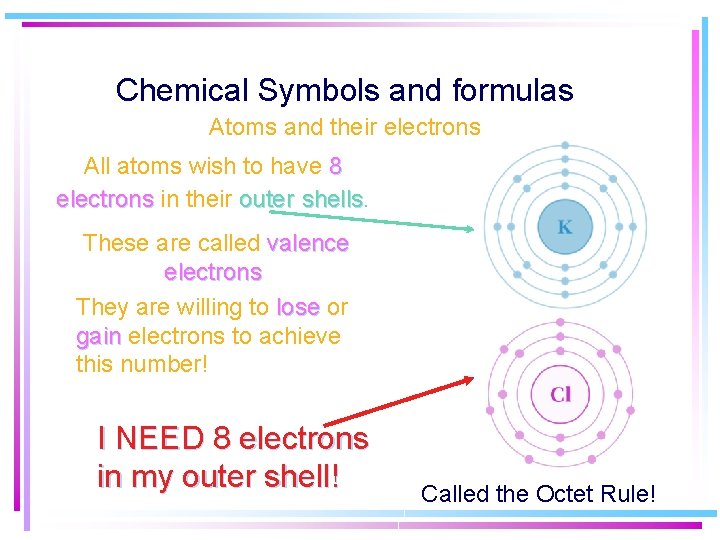
Chemical Symbols and formulas Atoms and their electrons All atoms wish to have 8 electrons in their outer shells These are called valence electrons They are willing to lose or gain electrons to achieve this number! I NEED 8 electrons in my outer shell! Called the Octet Rule!

Ions • An ion is an atom or group of atoms that has a charge • A compound that is composed of ions is called an ionic compound. • Ionic compounds usually form between a metal (cation) and a nonmetal (anion). (Look at Periodic Table) • They may also form between a polyatomic ion (like ammonium) and either a metal or nonmetal. • In ionic compounds, you will TRANSFER valence electrons
Ions • A cation – positive ion – formed when an atom loses electrons. • Example: Na+ • An anion – negative ion – is formed when an atom gains electrons • Example: Cl- • A monatomic ion is one element with a charge • A polyatomic ion is more than one element with a charge • Example: SO 42 -
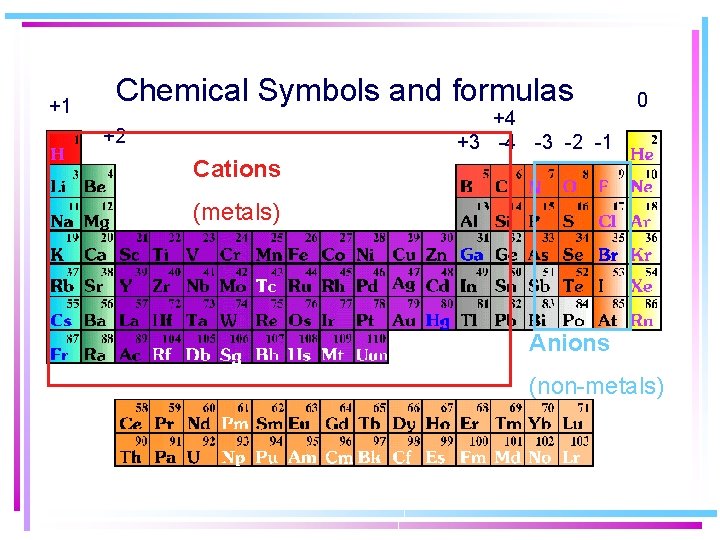
+1 Chemical Symbols and formulas +4 +3 -4 -3 -2 -1 +2 0 Cations (metals) Anions (non-metals)
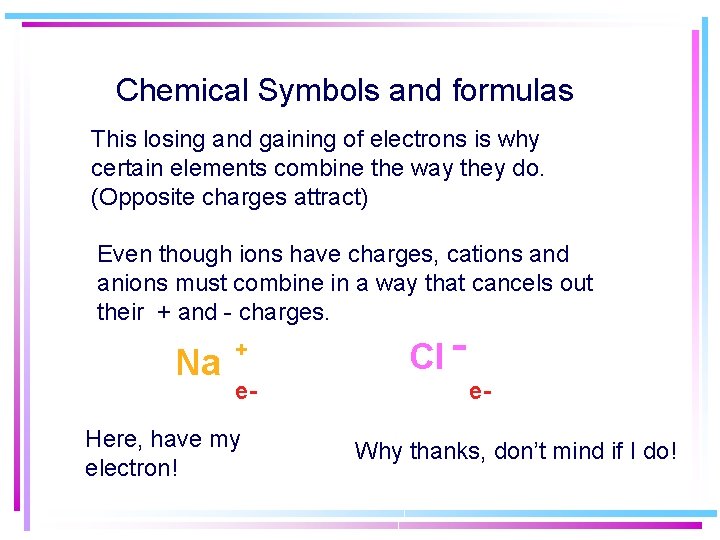
Chemical Symbols and formulas This losing and gaining of electrons is why certain elements combine the way they do. (Opposite charges attract) Even though ions have charges, cations and anions must combine in a way that cancels out their + and - charges. Na + e- Here, have my electron! Cl - e. Why thanks, don’t mind if I do!
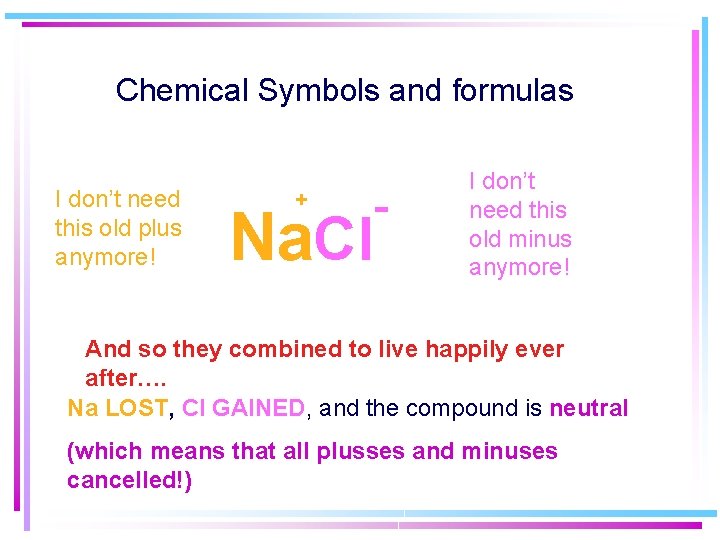
Chemical Symbols and formulas I don’t need this old plus anymore! + Na. Cl - I don’t need this old minus anymore! And so they combined to live happily ever after…. Na LOST, Cl GAINED, and the compound is neutral (which means that all plusses and minuses cancelled!)

Writing Ionic Compounds 1. Find cation (metal) and write symbol and charge 2. Find anion (nonmetal) and write symbol and charge - If ends in “ide” on periodic table - If end in “ate” on class copy sheet 3. Criss Cross from top to bottom 4. Charges cancel (remove + & -) 5. Simplify/reduce if possible *Don’t forget parentheses when you bring down anything other than a 1 with polyatomic ions. *For transition elements with varying charge, the roman numeral indicates the charge on the metal.

Formula for boron oxide 1. Find cation (metal) and write symbol and charge. 3+ B

Formula for boron oxide 2. Find anion (nonmetal) and write symbol and charge. Since it ends in “–ide” it is on the periodic table. 3+ B 2 O
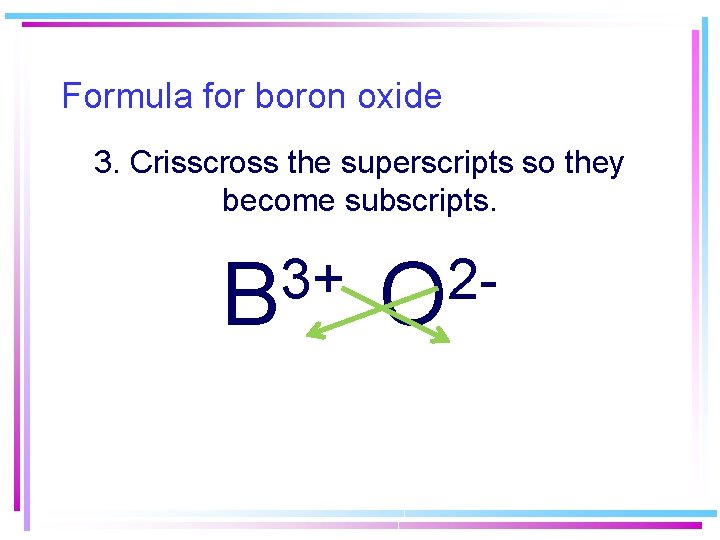
Formula for boron oxide 3. Crisscross the superscripts so they become subscripts. 3+ B 2 O

Formula for boron oxide 4. Remove charges (+ and – cancel) 5. Reduce subscripts when possible. (not possible here) B 2 O 3

Examples 1. 2. 3. 4. 5. Sodium & Chloride Magnesium & Bromide Calcium & Sulfide Sodium & Nitrate Ammonium and Sulfate

Naming Ionic Compounds (Regular & Polyatomic) 1. Write the name of the cation (metal) 2. Write the name if the anion (nonmetal) -If only 2 elements, cut off the ending of the 2 nd element and add “ide” -If a polyatomic ion, use the name the class copy data sheet gives you.
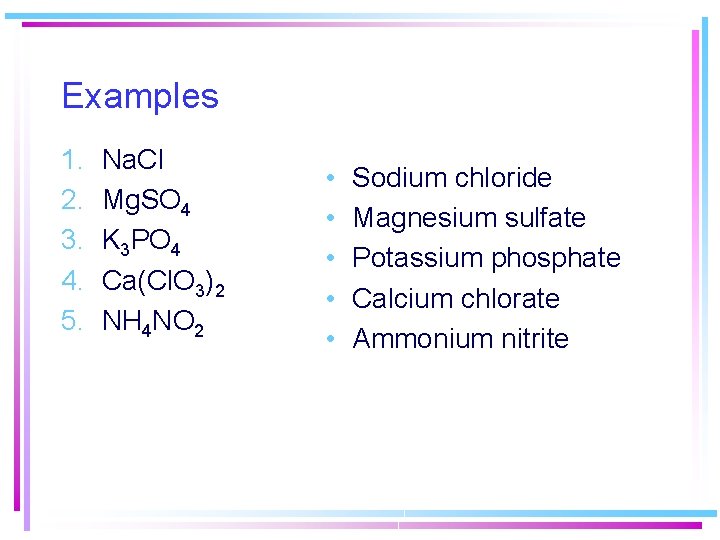
Examples 1. 2. 3. 4. 5. Na. Cl Mg. SO 4 K 3 PO 4 Ca(Cl. O 3)2 NH 4 NO 2 • • • Sodium chloride Magnesium sulfate Potassium phosphate Calcium chlorate Ammonium nitrite

Naming Ionic Compounds (Variable Charge) 1. Check to see that these metal are in the d-block and have a variable charge 2. Write the name of the cation (metal) with (roman numerals) 3. Write the name of the anion (nonmetal or polyatomic ion) 4. Add 1’s to elements with no #’s 5. Reverse criss cross and add positive charge to the metal and a negative charge to the nonmetal 6. Check the charge on nonmetal -If incorrect multiply both sides -# attached to the metal goes in the roman numeral
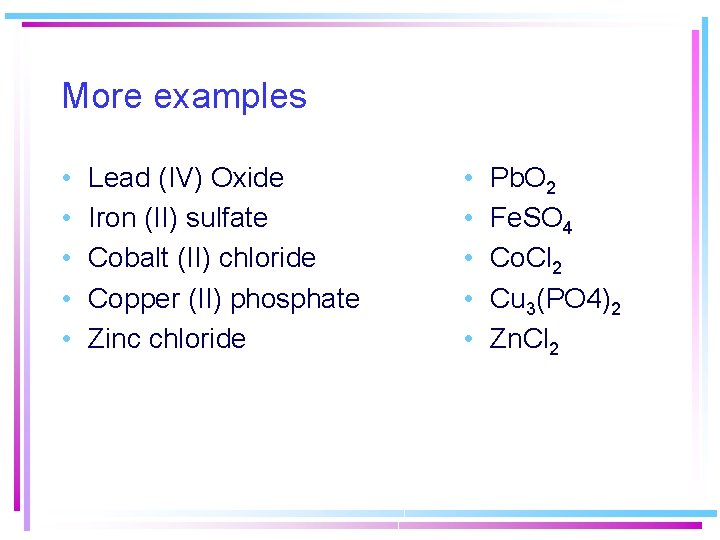
More examples • • • Lead (IV) Oxide Iron (II) sulfate Cobalt (II) chloride Copper (II) phosphate Zinc chloride • • • Pb. O 2 Fe. SO 4 Co. Cl 2 Cu 3(PO 4)2 Zn. Cl 2
- Slides: 24