Chemical Rxn Rates Chemical Kinetics The area of




- Slides: 22

Chemical Rxn Rates

Chemical Kinetics The area of chemistry that concerns reaction rates. Key Idea: Molecules must collide to react. However, only a small fraction of collisions produces a reaction. Why?

Collision Model Collisions must have enough energy to produce the reaction (must equal or exceed the activation energy). Orientation of reactants must allow formation of new bonds.

Reaction Rate l Speed at which a chemical reaction takes place l Reaction rate depends on the collisions between reacting particles. l Determined by measuring the change in concentration of a reactant or product per unit of time

Factors Affecting Reaction Rates l Temperature: Measure of average kinetic energy of the molecules in a substance l Concentration: amount of molecules present in a unit volume l Catalyst: an agent that speeds up the rate of chemical rxns w/out being permanently changed or used up l Surface area: area of the surface

Temperature l Molecules at higher temp. have higher KE and move faster more collisions faster rxn rate l A 10°C increase in Temp double the rate of rxn l Temp Collisions Rxn rate

Surface Area l high SA = fast rxn rate ¡more opportunities for collisions ¡Increase surface area by… lusing smaller particles ldissolving in water l SA Collisions Rxn Rate

Concentration l Increasing concentration increases chance of effective collisions faster rxn rate l Conc Collisions Rxn Rate

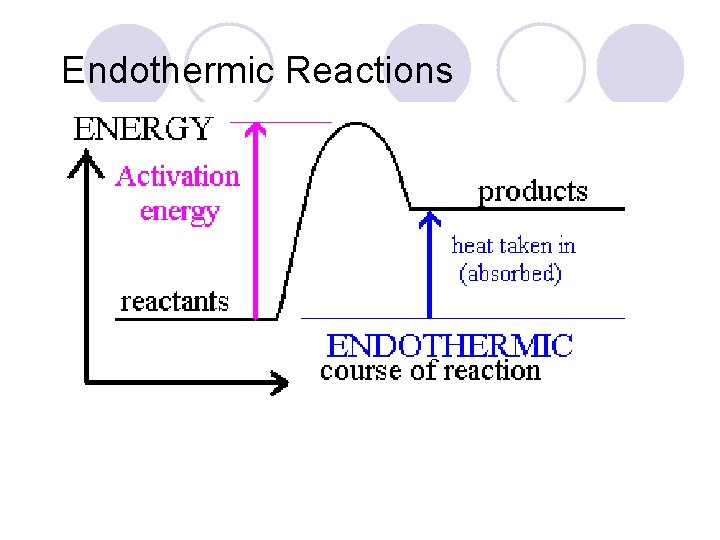
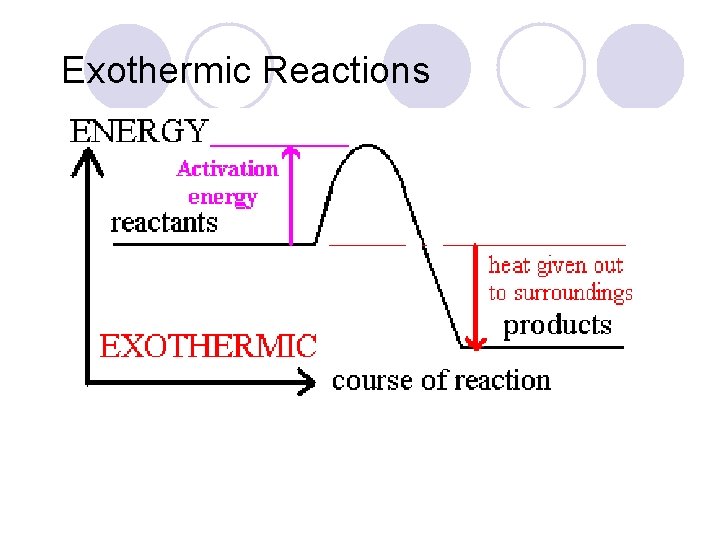
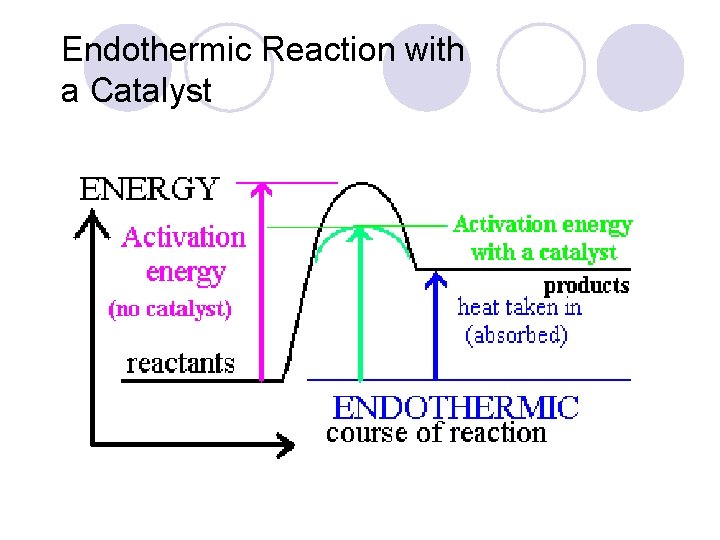
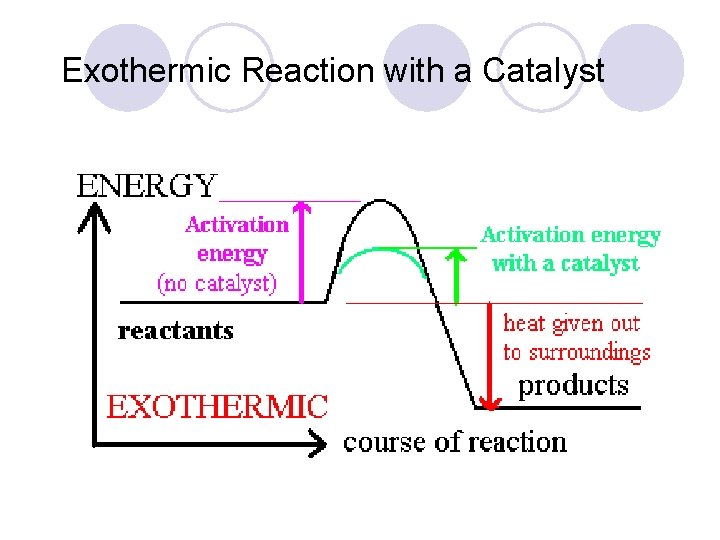
Heat of Reaction The amount of heat released or absorbed during a chemical reaction. Endothermic: Reactions in which energy is absorbed as the reaction proceeds. Exothermic: Reactions in which energy is released as the reaction proceeds.

Endothermic Reactions

Exothermic Reactions

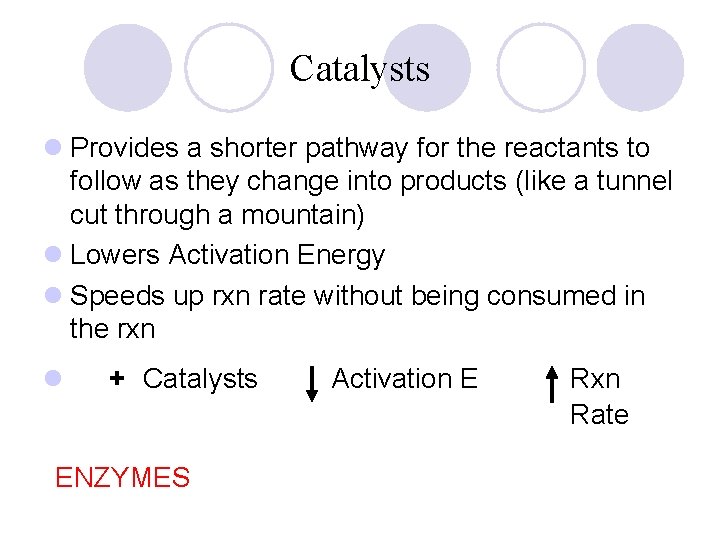
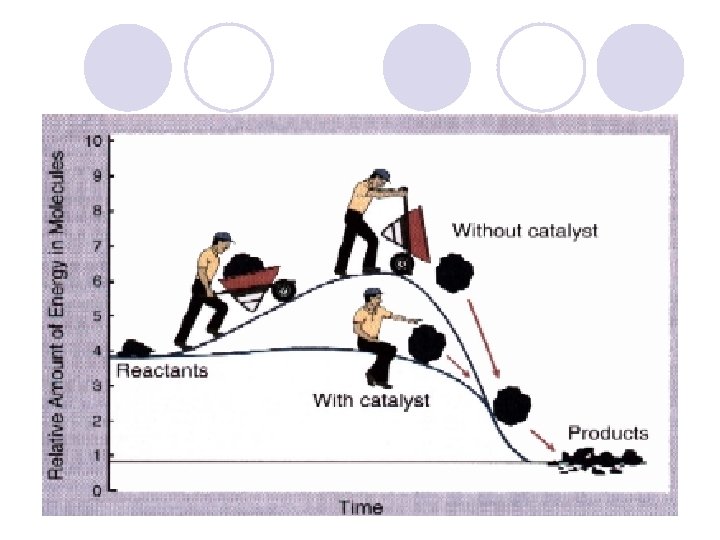
Catalysts l Provides a shorter pathway for the reactants to follow as they change into products (like a tunnel cut through a mountain) l Lowers Activation Energy l Speeds up rxn rate without being consumed in the rxn l + Catalysts ENZYMES Activation E Rxn Rate

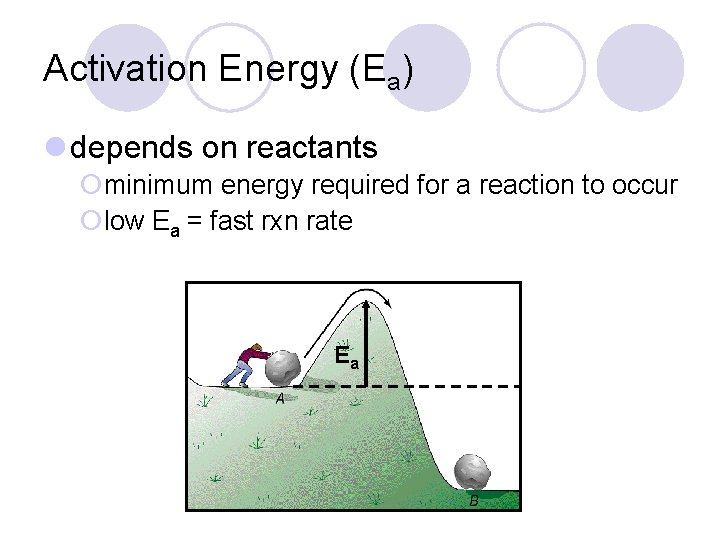
Activation Energy (Ea) l depends on reactants ¡minimum energy required for a reaction to occur ¡low Ea = fast rxn rate Ea


Endothermic Reaction with a Catalyst

Exothermic Reaction with a Catalyst

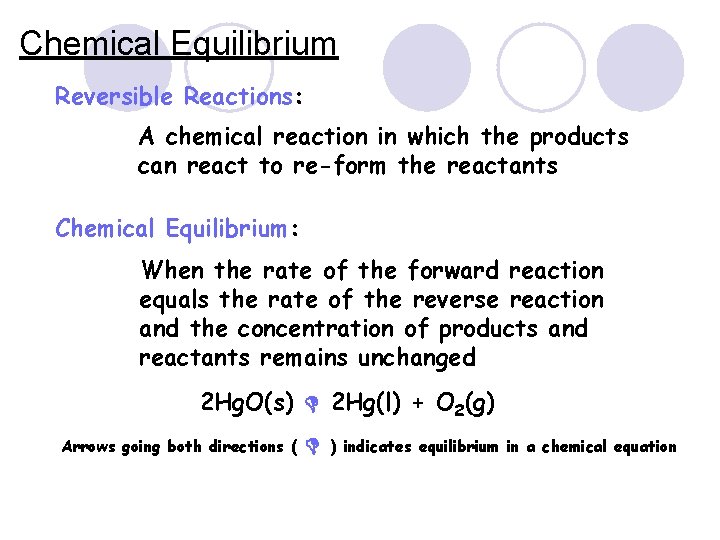
Chemical Equilibrium Reversible Reactions: A chemical reaction in which the products can react to re-form the reactants Chemical Equilibrium: When the rate of the forward reaction equals the rate of the reverse reaction and the concentration of products and reactants remains unchanged 2 Hg. O(s) 2 Hg(l) + O 2(g) Arrows going both directions ( ) indicates equilibrium in a chemical equation

Le. Chatelier’s Principle When a system at equilibrium is placed under stress, the system will undergo a change in such a way as to relieve that stress.

Le Chatelier Translated: When you take something away from a system at equilibrium, the system shifts in such a way as to replace what you’ve taken away. When you add something to a system at equilibrium, the system shifts in such a way as to use up what you’ve added.

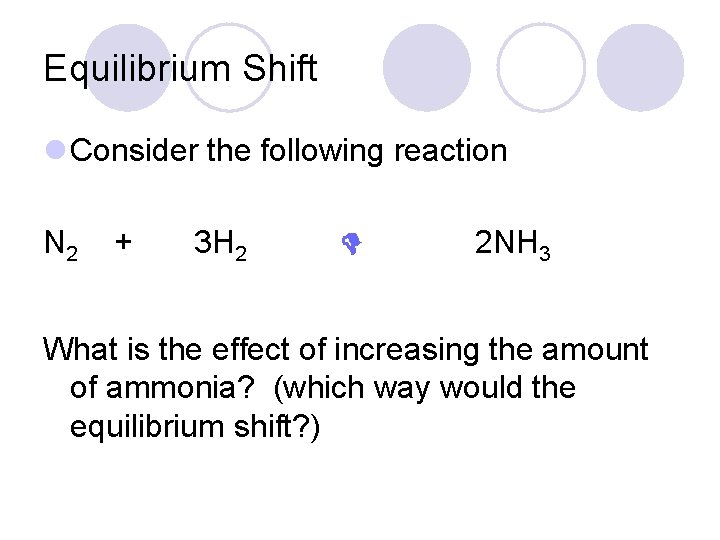
Equilibrium Shift l Consider the following reaction N 2 + 3 H 2 2 NH 3 What is the effect of increasing the amount of ammonia? (which way would the equilibrium shift? )

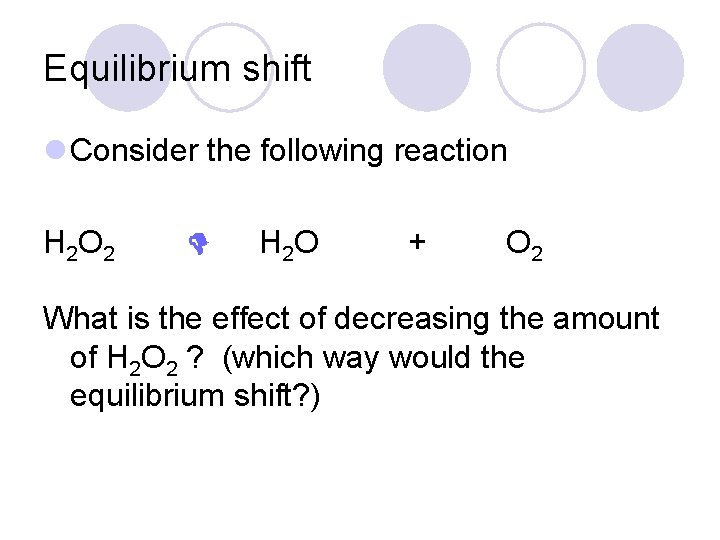
Equilibrium shift l Consider the following reaction H 2 O 2 H 2 O + O 2 What is the effect of decreasing the amount of H 2 O 2 ? (which way would the equilibrium shift? )

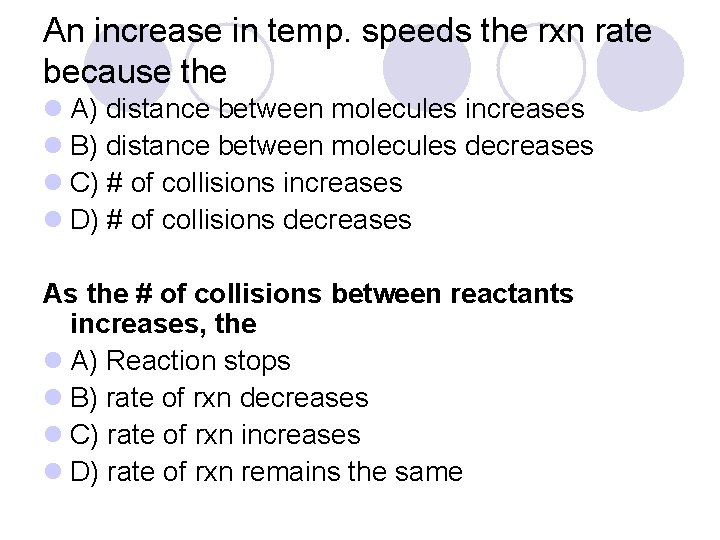
An increase in temp. speeds the rxn rate because the l A) distance between molecules increases l B) distance between molecules decreases l C) # of collisions increases l D) # of collisions decreases As the # of collisions between reactants increases, the l A) Reaction stops l B) rate of rxn decreases l C) rate of rxn increases l D) rate of rxn remains the same