Chemical Risk Assessment Exposure Monitoring Qualitative Chemical Risk















- Slides: 26

Chemical Risk Assessment & Exposure Monitoring Qualitative Chemical Risk Assessment Revision December 2010 - Information provided subject to the 'Conditions for Sharing Materials and Advice' -

Objectives n At the end of this training session you will: – Know the terminology associated with qualitative chemical risk assessments – Be able to identify chemical hazards – Be able to complete a Qualitative Exposure Assessment – Be able to develop Exposure Monitoring Priorities 2

What is a Risk Assessment? n It is a systematic identification: – of the hazards associated with an activity – and evaluation of the risk associated with this activity u the likelihood or probability of the hazard occurring u the severity of the consequences of the hazard n A proactive approach to preventing occupational injury, ill health and disease. 3

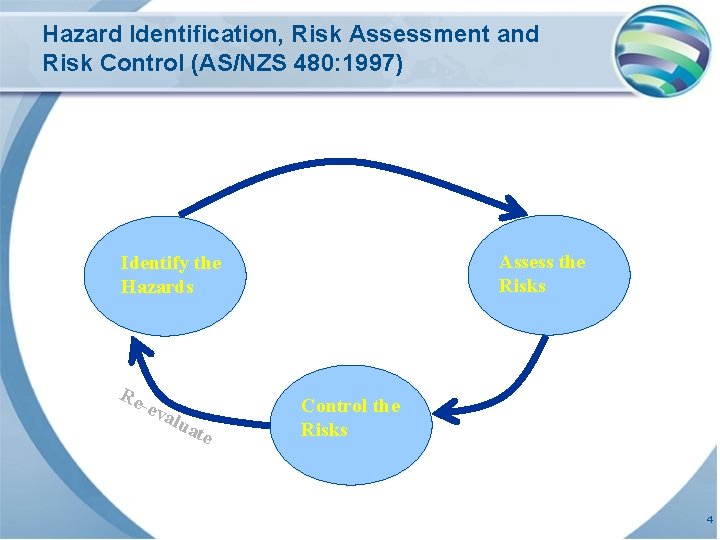
Hazard Identification, Risk Assessment and Risk Control (AS/NZS 480: 1997) Assess the Risks Identify the Hazards Re -ev alu ate Control the Risks 4

Definitions: Hazard and Risk n n Hazard - the potential for a chemical to produce adverse effects. (Toxicity) Risk - the probability that a hazard will be realized under certain conditions of use / exposure. Risk = Hazard X Exposure § § Safety = statement that a substance will NOT produce harm under specified conditions. Very toxic substances can be used safely provided one controls the environment to prevent absorption of quantities sufficient to produce toxicity. 5

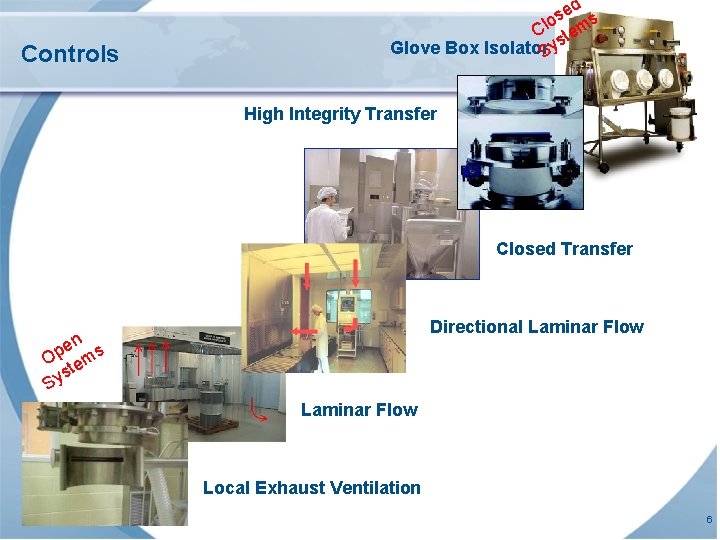
Controls ed s s o Cl tem Glove Box Isolator. Sys High Integrity Transfer Closed Transfer Directional Laminar Flow en s p O tem s Sy Laminar Flow Local Exhaust Ventilation 6

Industrial Hygiene Hazard Recognition n n What is the hazard? What is the task / activity? How much is used? How long is it used? How toxic is it? How dusty is it? How volatile is it? What type of hazard control is in place? How many people are exposed? How often are people exposed? 7

Industrial Hygiene Hazard Recognition n n How much is used? Milligrams, kilograms, tons? How long is the exposure? Minutes, hours, days, months, lifetime? What controls are used? a. Work practices? b. Ventilation? c. Enclosures? d. Administrative? e. Personal protective equipment? 14

WHERE DO I GET THAT INFORMATION? n Process Sheets n Safety Data Sheets n Workers n Observation n Supervisors n Engineers n Chemists n Your own company network 15

Qualitative Risk Assessment - Definition Qualitative Risk Assessment Definition Using professional experience judgment to rate the potential exposure based on the duration, magnitude of exposure and the agent toxicity, independent of personal protective equipment 16

Qualitative Risk Assessment - Purpose The Purpose of a Qualitative Assessment n Identify the Hazard n Anticipate/Estimate Severity of Exposure n Develop Exposure Monitoring Priorities to – Quantify Exposure – Confirm Severity Estimations 17

Qualitative Exposure Assessment n Basic Characterization - a process used to identify the agents handled by every employee in the workplace (see separate presentation - step 1. ) – A key element in the qualitative assessment n IH should assess employee exposure risk by considering: – – – Probability of exposure Frequency of exposure The PBOEL category Acute vs. chronic health effects Routes of exposure. 18

Qualitative Exposure Assessment n Qualitative Exposure Assessment (QEA) – a process to assess the risk of exposure to the agents for each job title/activity developed during the basic characterization. n At a minimum, the QEA should address the following: – – – Assessed agents (and/or additive effects) Quantity handled per shift or operation Exposure description and information supporting the severity choice Controls Exposure severity ranking (Detailed in next slide) Assessment date and assessor’s name QEA: related to the whole process or unit operation 19

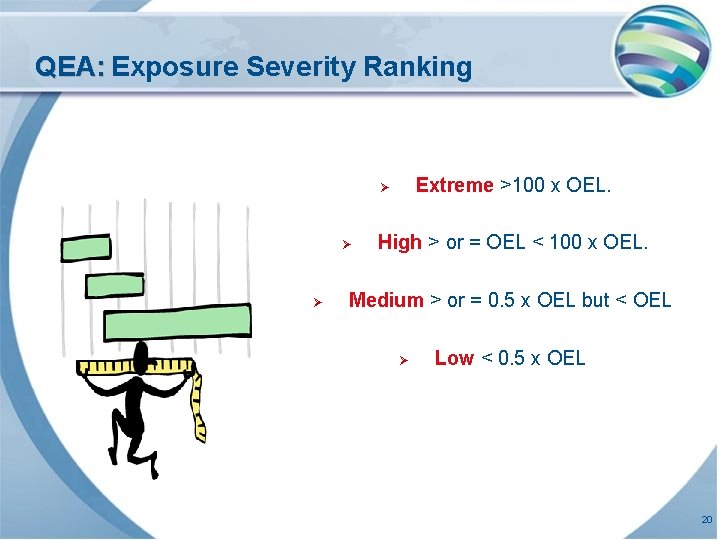
QEA: Exposure Severity Ranking Extreme >100 x OEL. Ø Ø Ø High > or = OEL < 100 x OEL. Medium > or = 0. 5 x OEL but < OEL Ø Low < 0. 5 x OEL 20

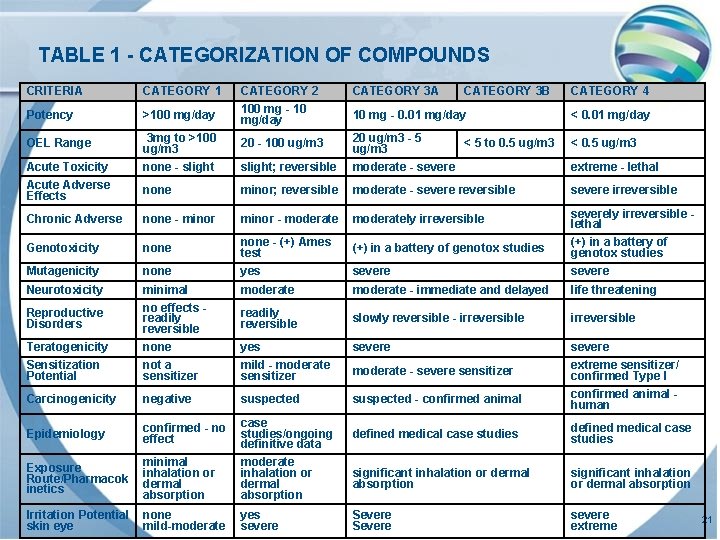
TABLE 1 - CATEGORIZATION OF COMPOUNDS CRITERIA CATEGORY 1 CATEGORY 3 A >100 mg/day CATEGORY 2 100 mg - 10 mg/day Potency 10 mg - 0. 01 mg/day < 0. 01 mg/day OEL Range 3 mg to >100 ug/m 3 20 - 100 ug/m 3 20 ug/m 3 - 5 ug/m 3 < 0. 5 ug/m 3 Acute Toxicity Acute Adverse Effects none - slight; reversible moderate - severe extreme - lethal none minor; reversible moderate - severe reversible severe irreversible Chronic Adverse none - minor - moderately irreversible severely irreversible lethal Genotoxicity none - (+) Ames test (+) in a battery of genotox studies Mutagenicity none yes severe Neurotoxicity moderate - immediate and delayed life threatening readily reversible slowly reversible - irreversible Teratogenicity Sensitization Potential minimal no effects readily reversible none not a sensitizer yes mild - moderate sensitizer severe moderate - severe sensitizer severe extreme sensitizer/ confirmed Type I Carcinogenicity negative suspected - confirmed animal human Epidemiology confirmed - no effect case studies/ongoing definitive data defined medical case studies Exposure Route/Pharmacok inetics minimal inhalation or dermal absorption moderate inhalation or dermal absorption significant inhalation or dermal absorption Irritation Potential skin eye none mild-moderate yes severe Severe severe extreme Reproductive Disorders CATEGORY 3 B < 5 to 0. 5 ug/m 3 CATEGORY 4 21

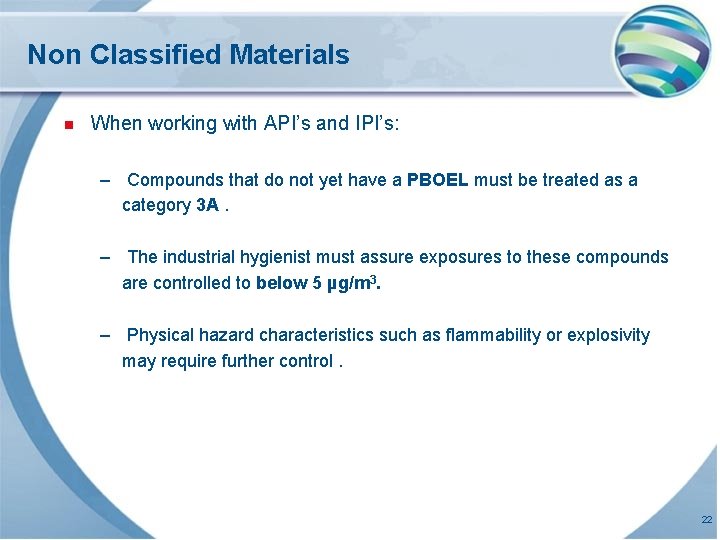
Non Classified Materials n When working with API’s and IPI’s: – Compounds that do not yet have a PBOEL must be treated as a category 3 A. – The industrial hygienist must assure exposures to these compounds are controlled to below 5 µg/m 3. – Physical hazard characteristics such as flammability or explosivity may require further control. 22

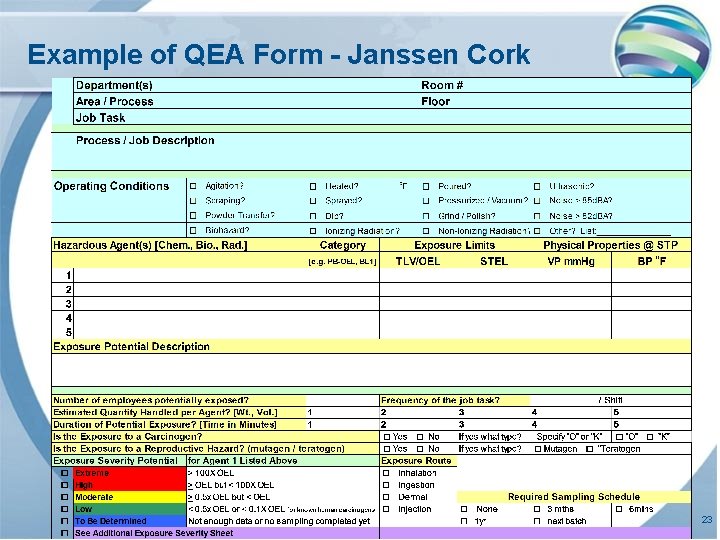
Example of QEA Form - Janssen Cork 23

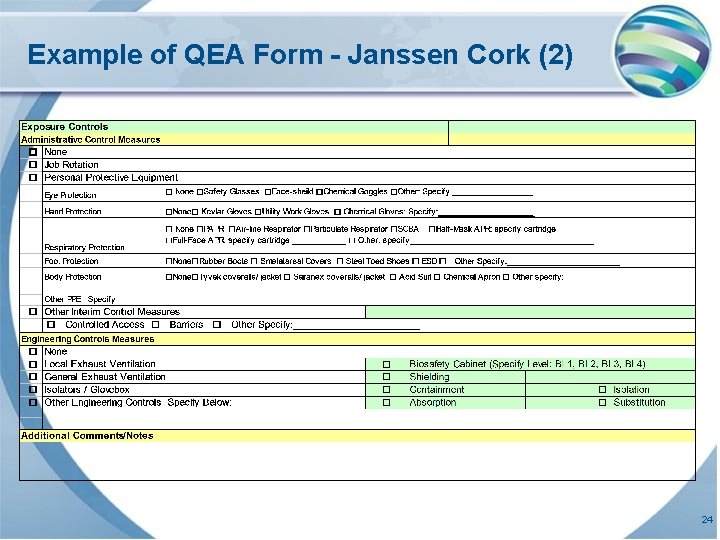
Example of QEA Form - Janssen Cork (2) 24

Example of QEA Form - RBEAP solvents Pharma 25

Example of QEA Form - RBEAP solvents Pharma (2) 26

Example of QEA Form - RBEAP API Pharma 27

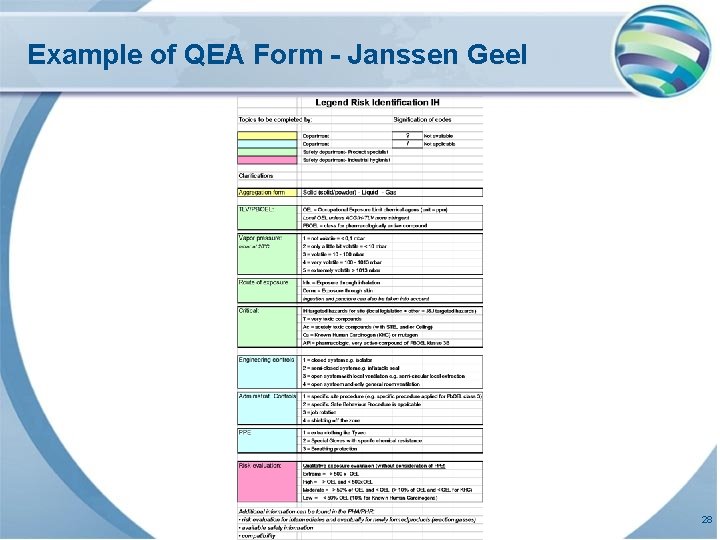
Example of QEA Form - Janssen Geel 28

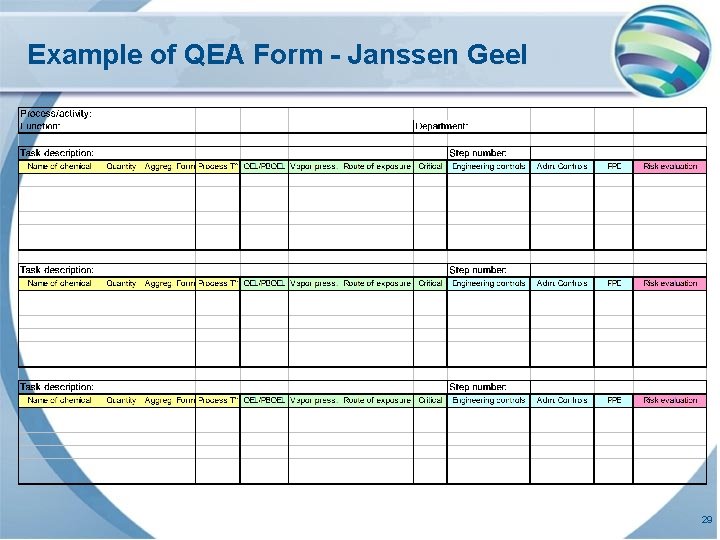
Example of QEA Form - Janssen Geel 29

QEA: Outcome - WHAT DO I DO NOW? Remark December 2010: The approach described in this slide is no longer applied in J&J at this time and must be considered to be an example only Following the QEA the SEG/ Job titles /activities are risk ranked and the monitoring priorities are outlined below: Priority Extreme Monitor ASAP - begin engineering controls immediately High sample next - within 3 months Medium sample next - within 3 -6 months Low No need to sample Except if : PBOEL 3 B or 4 Acute toxicants Known Human carcinogens ideally sample within 12 months. 30

Exposure Monitoring Priorities As a result of the QEA, Exposure Monitoring Priorities are established, that include: n All areas/tasks that require sampling. – The type of sampling to be conducted Personal exposure sampling during activity – Full Shift if appropriate – Task (partial shift) u STEL if applicable u General area u Wipe samples if applicable u – Sampling frequency - based on Exposure Severity Rating 31

Recap n Definitions : – Hazard - the potential for a chemical to produce adverse effects. (Toxicity) – Risk - the probability that a hazard will be realized under certain conditions of use / exposure. n The Purpose of a Qualitative Exposure Assessment – Identify the Hazard – Anticipate/Estimate Severity of Exposure – Develop Exposure Monitoring Priorities n The Exposure Monitoring Priorities shall identify All areas/tasks that require sampling. – Specify the type of sampling to be conducted Employee exposure / Full Shift / Task (partial shift) / STEL u General area u – Sampling frequency 33