Chemical reactions6 2 Identify the parts of a



















- Slides: 28

Chemical reactions-6. 2 Identify the parts of a chemical reaction

Chemical v. Physical • Is boiling water an example of a chemical reaction? • Demonstrate chemical v. Physical change • 3 pieces of paper: Identify the change? a) Burned corners b) Cut corners c) Hole in the middle

3 -column Notes • Chemical reaction, reactant, product, activation energy, catalyst, enzyme, substrate, active site • term→ prediction → definition → example

Chemical reactions • WHAT IS THE RELATIONSHIP BETWEEN A CHEMICAL BOND A CHEMICAL REACTIONS?

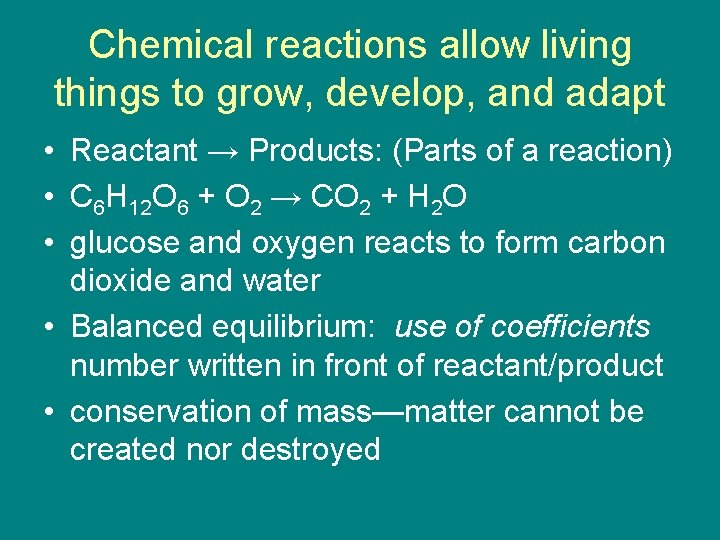
Chemical reactions allow living things to grow, develop, and adapt • Reactant → Products: (Parts of a reaction) • C 6 H 12 O 6 + O 2 → CO 2 + H 2 O • glucose and oxygen reacts to form carbon dioxide and water • Balanced equilibrium: use of coefficients number written in front of reactant/product • conservation of mass—matter cannot be created nor destroyed

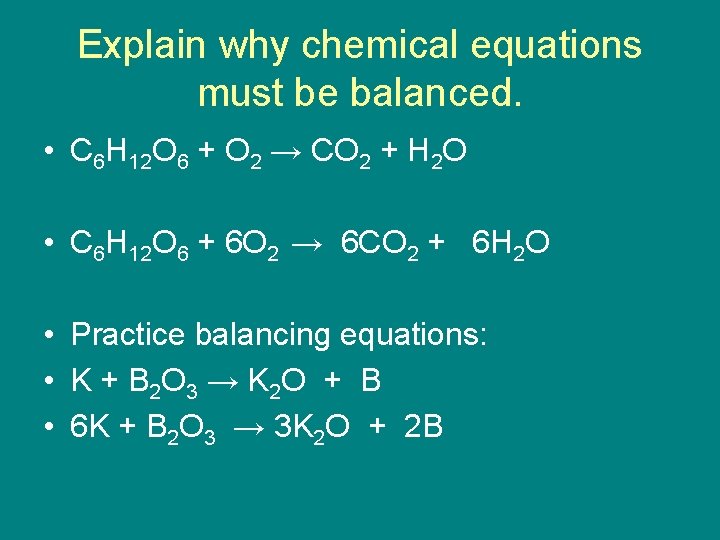
Explain why chemical equations must be balanced. • C 6 H 12 O 6 + O 2 → CO 2 + H 2 O • C 6 H 12 O 6 + 6 O 2 → 6 CO 2 + 6 H 2 O • Practice balancing equations: • K + B 2 O 3 → K 2 O + B • 6 K + B 2 O 3 → 3 K 2 O + 2 B

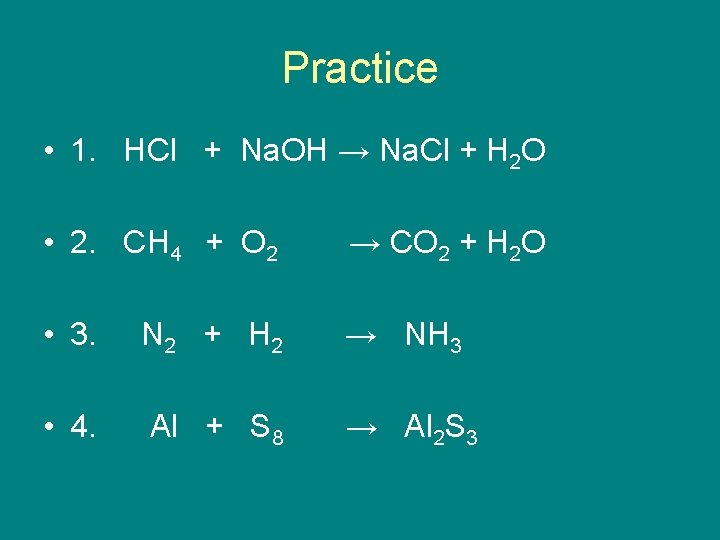
Practice • 1. HCl + Na. OH → Na. Cl + H 2 O • 2. CH 4 + O 2 → CO 2 + H 2 O • 3. N 2 + H 2 → NH 3 • 4. Al + S 8 → Al 2 S 3

Answers • 1. HCl + Na. OH → Na. Cl + H 2 O • 2. CH 4 + 2 O 2 → CO 2 + 2 H 2 O • 3. → 2 NH 3 N 2 + 3 H 2 • 4. 16 Al + 3 S 8 → 8 Al 2 S 3

Activation energy • The minimum amount of energy needed for reactants to form products in a chemical reaction. • Figure 6. 15 and 6. 16 -skill practice-visual literacy- read text under Activation energy and enzymes.

Activation Energy • Consider how fig. 6. 17 depicts key concepts. Draw graphs showing effect of a catalyst on the activation energy needed for a chemical reaction.

• What are enzymes? • Why are enzymes important to living things? • Name some biological processes that require enzymatic activity. • What effect does an enzyme have on a chemical reaction?

Key and Lock • How do enzymes maintain specificity? • Evaluate: formative assessment • Relate this concept to the analogy “key and lock”. • List the factors that may alter the activity of an enzyme. • Enzymes: describe the conditions inside the cell that enable enzymes to be active.

Water and Solution 6. 3 • The properties of water make it well suited to help maintain homeostasis in an organism. • OBJECTIVE: • Compare and contrast solutions and suspensions. • Describe the difference between acids and bases

1 st Objective • Evaluate how the structure of water makes it a good solvent. • Physical property: characteristic of matter, such as color or melting point, that can be observed or measured changing the composition of the substance.

3 -Column Notes • Polar molecule, hydrogen bond, mixture, solution, solvent, solute acid, base, p. H, buffer. • term→ prediction → definition → example

Water Properties • Polar molecule-what does it mean to have polarity? • Adhesion—able to stick to surfaces • Cohesion-able to stick to itself • High specific heat-moderates temperature • Floats when it freezes-expands • Versatile solvent

Morphological Word Analogy • Homogeneous v. heterogeneous • Predict the meaning of these terms • Use the Internet to search for these terms and write down some of what you find. • Brainstorm examples of each term • Discuss the meaning of these terms

Acids and Bases • Acids: on a p. H scale 0— 6, (7—neutral) • Bases: on a p. H scale 8— 14 • Data Analysis Lab 6. 1, pp. 164 complete. • p. H and buffers-balance solutions to maintain equilibrium= strong acid + strong base= a salt + water

The Building Blocks of Life • Organic Chemistry- compounds containing C. H. O in a 1: 2: 1 ratio- see glucose • Inorganic Chemistry-compounds not containing C, H, O in a 1: 2: 1 ratio like hydrogen carbons • Four macromolecules are organic • Carbohydrates, proteins, lipids, nucleic acids (DNA→RNA)

What does it mean to be organic? • Objectives: describe the role of carbon in living organisms. Summarize the four major families of biological macromolecules. Compare the functions of each group of macromolecules. • Terms: macromolecules, polymer, lipids, carbohydrates, proteins, amino acids, Nucleotides.

Complete the table • Chemical/ symbol: carbon/ C, hydrogen, oxygen, nitrogen, phosphorous, sulfur. • Atomic Number: 6 • Atomic Mass: 12 • Bonds Formed: 4

Clarify Misconception • If a vegetable farmer grows crops without the use of chemical fertilizers and pesticides, is the produce considered organic? • Organic molecules: • Organic foods

Differentiate • Starch is a polysaccharide, dissolves easily in water, whereas cellulose does not. Both molecules consists of polymers of glucose molecules (sugar). • What structural difference between starch and cellulose accounts for their difference in solubility?

Lipids • Saturated fats: • Unsaturated fats: • Phospholipids: • Steroids:

Anticipation Guide • Before reading about Proteins: Predict T/F • • 1. proteins are organic molecules 2. proteins are made in cells 3. enzymes are proteins 4. amino acids are the building blocks of proteins

Clarify misconceptions • What types of food other than meats are rich source of proteins? • 20 different amino acids occurs in a triple code—a codon. • Protein structurally in primary, secondary, tertiary, quaternary,

Nucleic acids DNA/ RNA • Nucleotides—subunits • DNA’s sugar-Deoxyribose • RNA’s sugar---Ribose • Nitrogenous base—purines and pyrimidine Purines—Adenine and Guanine Pyrimidine---Thymine and Cytosine Bonds A—T and G—C

Formative assessment • What is the relationship between macromolecules and polymers? • What are the four categories of macromolecules? • Study for chapter 6 test on Wednesday.