Chemical Reactions What makes what When do we














- Slides: 33

Chemical Reactions What makes what? When do we know when we are seeing one? …

The Nature of Chemical Reactions Chemical reactions change substances – Familiar examples: grow, ripen, decay, burn, respiration, digestion, photosynthesis… – Production of gas, color change, new products, and change in temperature Chemical reactions re-arrange atoms – Matter is not destroyed, just rearranged – Reactants make the products. Look for the → Isooctane (C 8 H 18) + Oxygen (O 2) → CO 2 + H 2 O The arrow points to the new products from the reactants

Signs of Chemical Reactions There are five main signs that indicate a chemical reaction has taken place: release input change in color change in odor production of new gases or vapor input or release of energy difficult to reverse

How Reactions Occur Collisions – For a chemical reaction to occur, the atoms/molecules must actually come in contact with another substance Atoms collide into one another causing a chemical change and formation of new products This will be addressed later in this unit

Energy and Reactions Energy can be found in various forms: – heat, electricity, light, chemical (nuclear) & mechanical Energy must be added to break the old bonds so that new bonds may be created – When the new bonds form, energy is released Other reactions may produce electricity or other energy forms Energy is conserved in chemical reactions – The total energy before the reaction must be the same energy of the products and their surroundings

Giving and Taking Energy Reactions may be exothermic or endothermic – Exothermic means releasing chemical energy Things get/feel warmer – Endothermic means absorbing Things get/feel cooler Energy may be added from other sources – heat for cooking, sunlight for photosynthesis, … Endergonic = endothermic Exergonic = exothermic

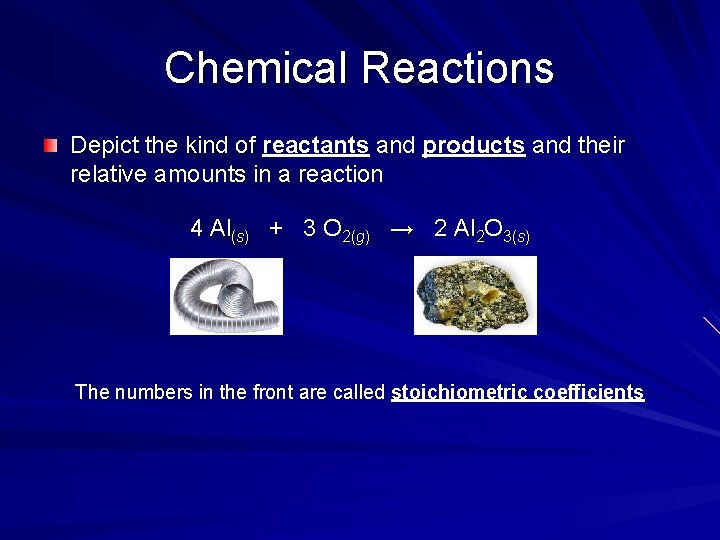
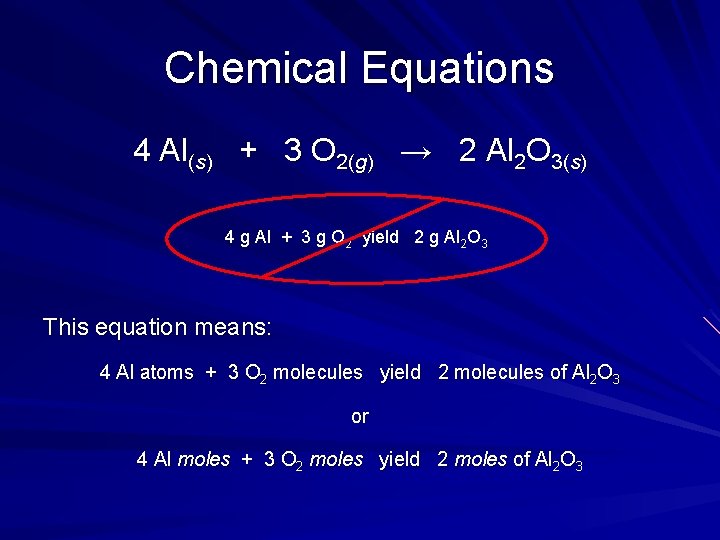
Chemical Reactions Depict the kind of reactants and products and their relative amounts in a reaction 4 Al(s) + 3 O 2(g) → 2 Al 2 O 3(s) The numbers in the front are called stoichiometric coefficients

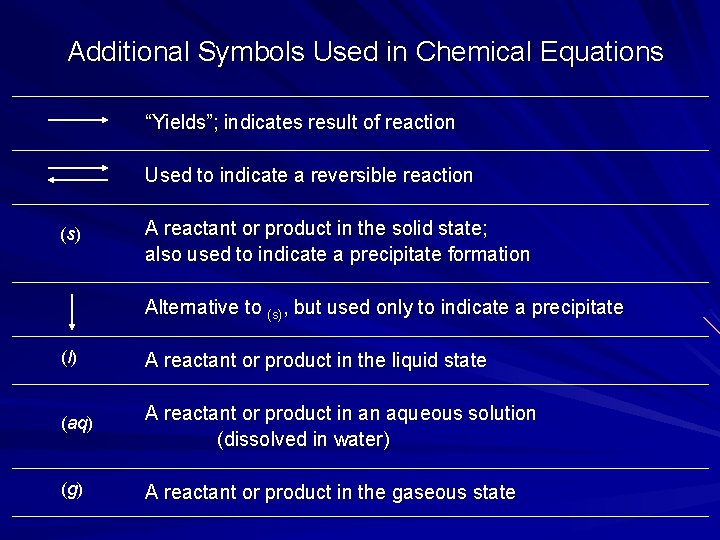
Additional Symbols Used in Chemical Equations “Yields”; indicates result of reaction Used to indicate a reversible reaction (s) A reactant or product in the solid state; also used to indicate a precipitate formation Alternative to (s), but used only to indicate a precipitate (l) A reactant or product in the liquid state (aq) A reactant or product in an aqueous solution (dissolved in water) (g) A reactant or product in the gaseous state

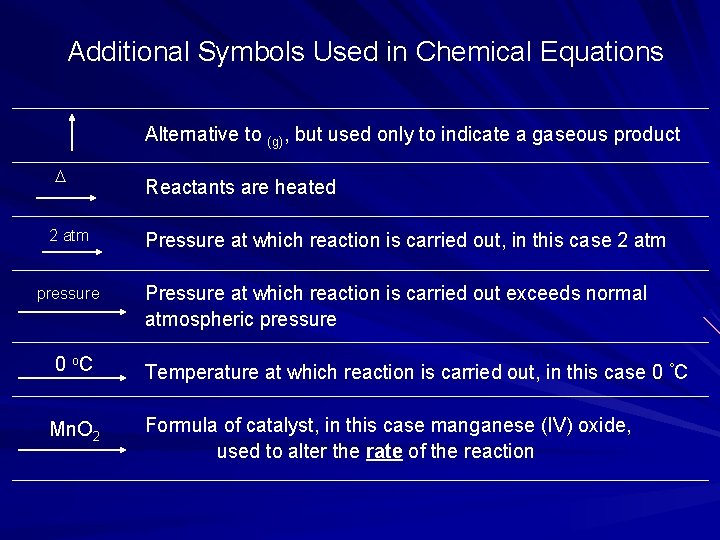
Additional Symbols Used in Chemical Equations Alternative to (g), but used only to indicate a gaseous product D 2 atm pressure Reactants are heated Pressure at which reaction is carried out, in this case 2 atm Pressure at which reaction is carried out exceeds normal atmospheric pressure 0 o. C Temperature at which reaction is carried out, in this case 0 °C Mn. O 2 Formula of catalyst, in this case manganese (IV) oxide, used to alter the rate of the reaction

Chemical Equations 4 Al(s) + 3 O 2(g) → 2 Al 2 O 3(s) 4 g Al + 3 g O 2 yield 2 g Al 2 O 3 This equation means: 4 Al atoms + 3 O 2 molecules yield 2 molecules of Al 2 O 3 or 4 Al moles + 3 O 2 moles yield 2 moles of Al 2 O 3

Word Equations A WORD EQUATION describes chemical change using the names of the reactants and products Write the word equation for the reaction of methane gas with oxygen gas yielding carbon dioxide and water. Methane + oxygen → carbon dioxide + water Reactants CH 4(g) + 2 O 2(g) → Products CO 2(g) + 2 H 2 O (g )

The Five Basic Types of Reactions 1. Synthesis 2. Decomposition 3. Combustion 4. Single-Displacement or “single-replacement” 5. Double-Displacement or “double-replacement” – Acid-Base Reaction: Often labeled as a type of reaction as it is a specific type of double-displacement

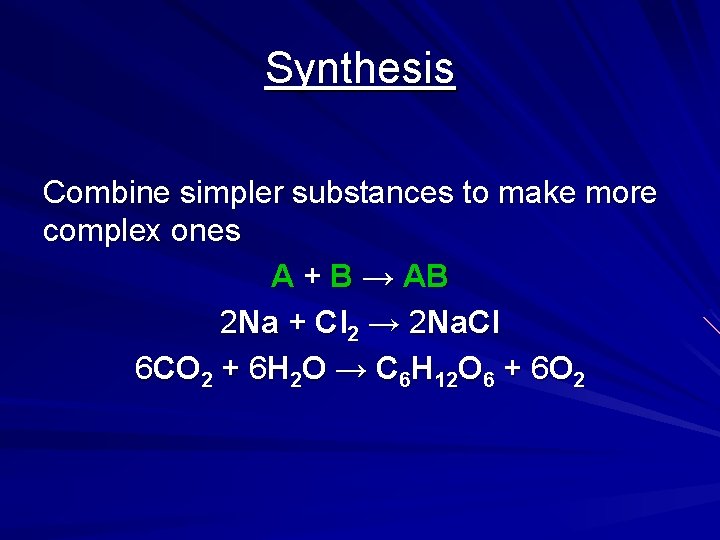
Synthesis Combine simpler substances to make more complex ones A + B → AB 2 Na + Cl 2 → 2 Na. Cl 6 CO 2 + 6 H 2 O → C 6 H 12 O 6 + 6 O 2

Decomposition Break complex substances into simpler ones AB → A + B 2 H 2 O → 2 H 2 + O 2 • You are looking for: • one reactant • a catalyst (heat or something on the yield sign) • small gas molecules in the products (H 2, O 2, etc. )

Combustion Uses oxygen (O 2) as a reactant, normally with a hydrocarbon 2 CH 4 + 4 O 2 → 2 CO 2 + 4 H 2 O excellent oxygen supply means cleaner “burning” 2 CH 4 + 3 O 2 → 2 CO + 4 H 2 O limited oxygen supply means a messier “burn” 2 CH 4 + 2 O 2 → 2 C + 4 H 2 O poor oxygen supply means a dirty “burn”

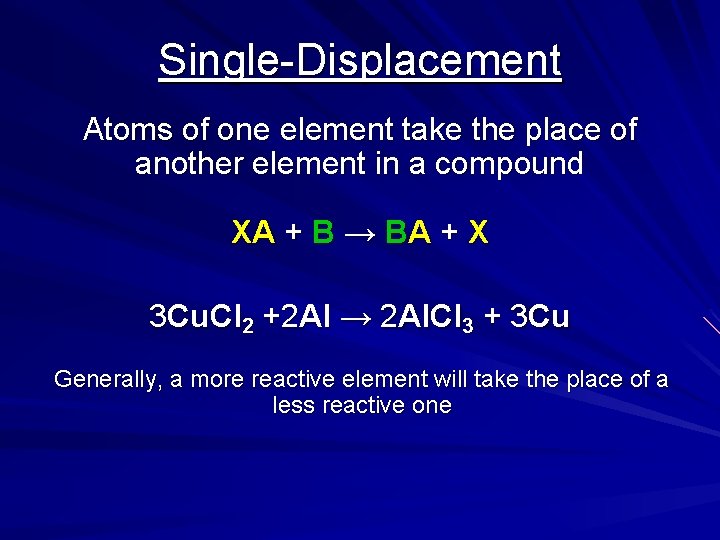
Single-Displacement Atoms of one element take the place of another element in a compound XA + B → BA + X 3 Cu. Cl 2 +2 Al → 2 Al. Cl 3 + 3 Cu Generally, a more reactive element will take the place of a less reactive one

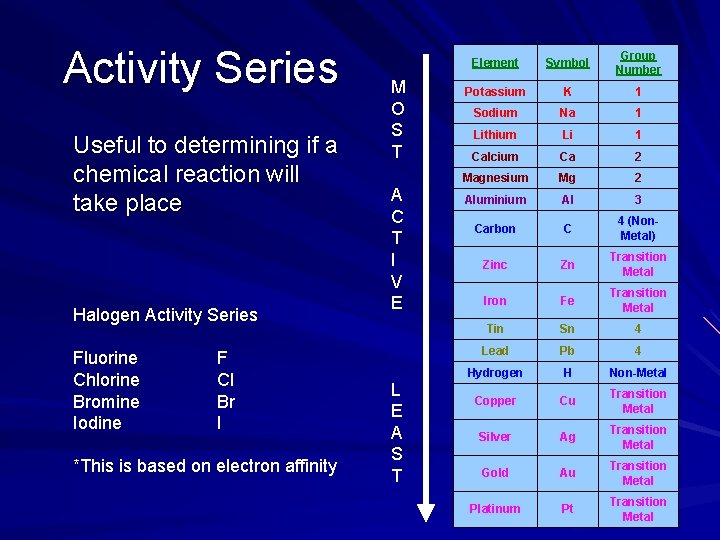
Activity Series Useful to determining if a chemical reaction will take place Halogen Activity Series Fluorine Chlorine Bromine Iodine F Cl Br I *This is based on electron affinity M O S T A C T I V E L E A S T Element Symbol Group Number Potassium K 1 Sodium Na 1 Lithium Li 1 Calcium Ca 2 Magnesium Mg 2 Aluminium Al 3 Carbon C 4 (Non. Metal) Zinc Zn Transition Metal Iron Fe Transition Metal Tin Sn 4 Lead Pb 4 Hydrogen H Non-Metal Copper Cu Transition Metal Silver Ag Transition Metal Gold Au Transition Metal Platinum Pt Transition Metal

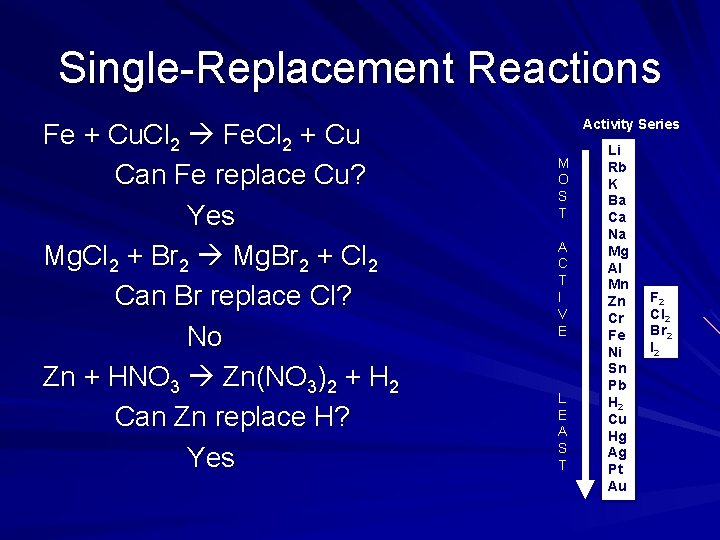
Single-Replacement Reactions Fe + Cu. Cl 2 Fe. Cl 2 + Cu Can Fe replace Cu? Yes Mg. Cl 2 + Br 2 Mg. Br 2 + Cl 2 Can Br replace Cl? No Zn + HNO 3 Zn(NO 3)2 + H 2 Can Zn replace H? Yes Activity Series M O S T A C T I V E L E A S T Li Rb K Ba Ca Na Mg Al Mn Zn Cr Fe Ni Sn Pb H 2 Cu Hg Ag Pt Au F 2 Cl 2 Br 2 I 2

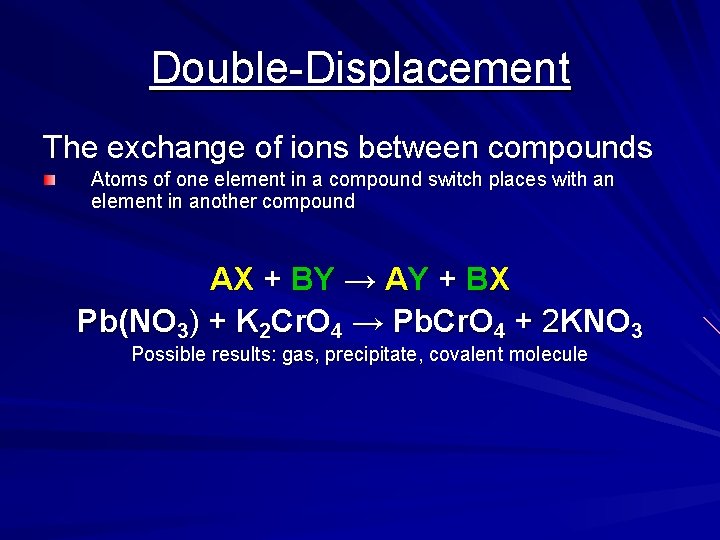
Double-Displacement The exchange of ions between compounds Atoms of one element in a compound switch places with an element in another compound AX + BY → AY + BX Pb(NO 3) + K 2 Cr. O 4 → Pb. Cr. O 4 + 2 KNO 3 Possible results: gas, precipitate, covalent molecule

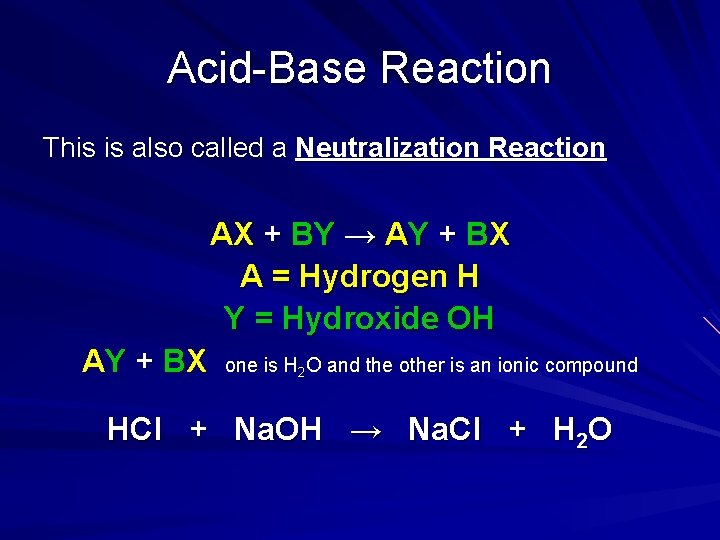
Acid-Base Reaction This is also called a Neutralization Reaction AX + BY → AY + BX A = Hydrogen H Y = Hydroxide OH AY + BX one is H 2 O and the other is an ionic compound HCl + Na. OH → Na. Cl + H 2 O

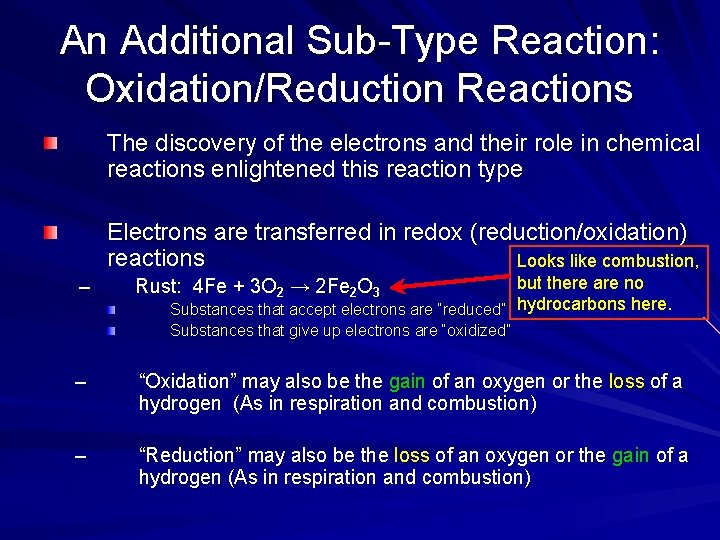
An Additional Sub-Type Reaction: Oxidation/Reduction Reactions The discovery of the electrons and their role in chemical reactions enlightened this reaction type Electrons are transferred in redox (reduction/oxidation) reactions Looks like combustion, – but there are no Substances that accept electrons are “reduced” hydrocarbons here. Rust: 4 Fe + 3 O 2 → 2 Fe 2 O 3 Substances that give up electrons are “oxidized” – “Oxidation” may also be the gain of an oxygen or the loss of a hydrogen (As in respiration and combustion) – “Reduction” may also be the loss of an oxygen or the gain of a hydrogen (As in respiration and combustion)

Na 2 S + Zn(NO 3)2 → Zn. S + 2 Na. NO 3 Na has been oxidized – Na begins bonded to S, no O’s – As a product, Na is bonded to O (along with other elements) Zn has been reduced – Zn begins by being bonded to O (along with other elements) – As a product, Zn has lost the O

Why do redox reactions occur? “Free radicals” – These are fragments of a molecule with at least one electron available for bonding – Similar to valence e-, but connected to molecules instead of atoms Making of polymers, combustion of rocket fuel, the burning of coal or oil all involve the formation of radicals

Balancing Chemical Reactions Follow the Law of Conservation of Mass (already covered) Balancing is done with coefficients, not subscripts. – Changing subscripts will change the substances – CH 4 + 2 O 2 → CO 2 + 2 H 2 O So? What does it mean? – Methane requires 2 oxygen molecules to combust and produce carbon dioxide and water, any less would not yield the same products, or it simply would not react. – AND, each burned methane yields 1 molecule of carbon dioxide and 2 molecules of water, if supplied with enough oxygen.

Coefficients Molecules is self explanatory, but can you “see” 1 or 2 molecules of anything? – Mole ratio (a. k. a. molar ratio) is just the coefficients – For 2 Mg + O 2 → 2 Mg. O; the ratio is 2: 1: 2 The moles for the above equation are as follows: – – – Remember to use the atomic masses as grams. Mg = 24. 3 g/mol, so 2 Mg = 48. 6 g O = 16. 0 g/mol, so O 2 = 32. 0 g (subscripts work the same way for determining masses. ) – 2 Mg. O = 80. 6 g, or Mg. O = 40. 3 g/mol

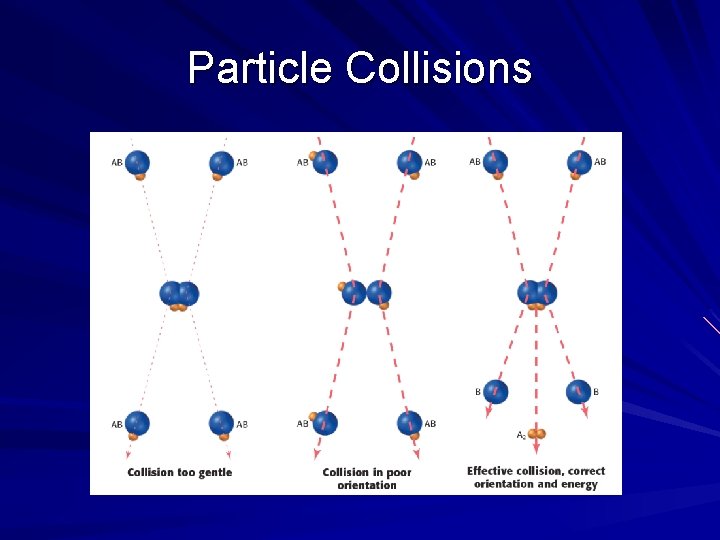
How Reactions Occur The Collision Theory – For a chemical reaction to occur, the atoms/molecules of each substance must actually come in contact with one other Thus, they collide causing a chemical change and formation of new products

Orientation Compounds are three-dimensional objects made of tiny microscopic atoms – When they collide, they have to hit with the correct orientation to transfer electrons, atoms or even synthesis a larger product A good analogy would be two basketball players attempting to pass the ball between them. If one was facing the wrong way, he/she would never be able to receive the pass (wrong orientation)

Particle Collisions

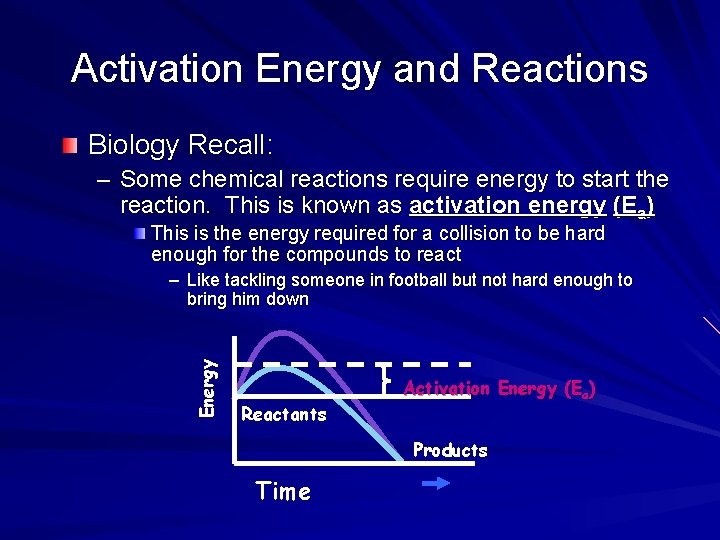
Activation Energy and Reactions Biology Recall: – Some chemical reactions require energy to start the reaction. This is known as activation energy (Ea) This is the energy required for a collision to be hard enough for the compounds to react Energy – Like tackling someone in football but not hard enough to bring him down Reactants Activation Energy (Ea) Products Time

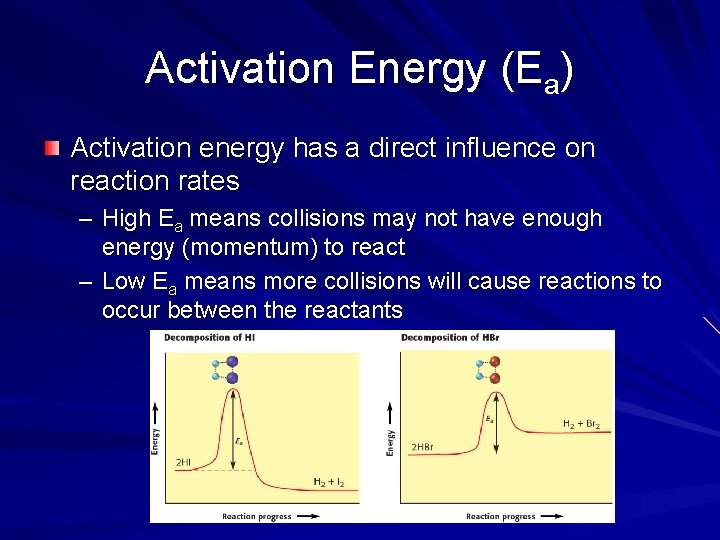
Activation Energy (Ea) Activation energy has a direct influence on reaction rates – High Ea means collisions may not have enough energy (momentum) to react – Low Ea means more collisions will cause reactions to occur between the reactants

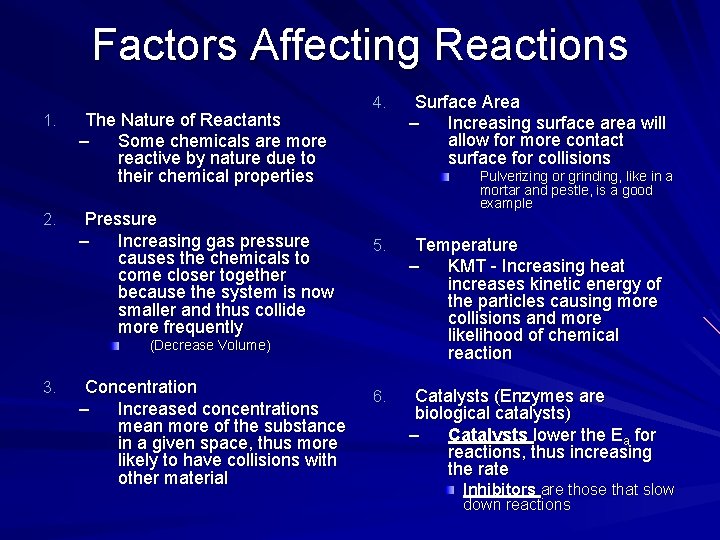
Factors Affecting Reactions 1. 2. The Nature of Reactants – Some chemicals are more reactive by nature due to their chemical properties Pressure – Increasing gas pressure causes the chemicals to come closer together because the system is now smaller and thus collide more frequently 4. Pulverizing or grinding, like in a mortar and pestle, is a good example 5. Temperature – KMT - Increasing heat increases kinetic energy of the particles causing more collisions and more likelihood of chemical reaction 6. Catalysts (Enzymes are biological catalysts) – Catalysts lower the Ea for reactions, thus increasing the rate (Decrease Volume) 3. Concentration – Increased concentrations mean more of the substance in a given space, thus more likely to have collisions with other material Surface Area – Increasing surface area will allow for more contact surface for collisions Inhibitors are those that slow down reactions

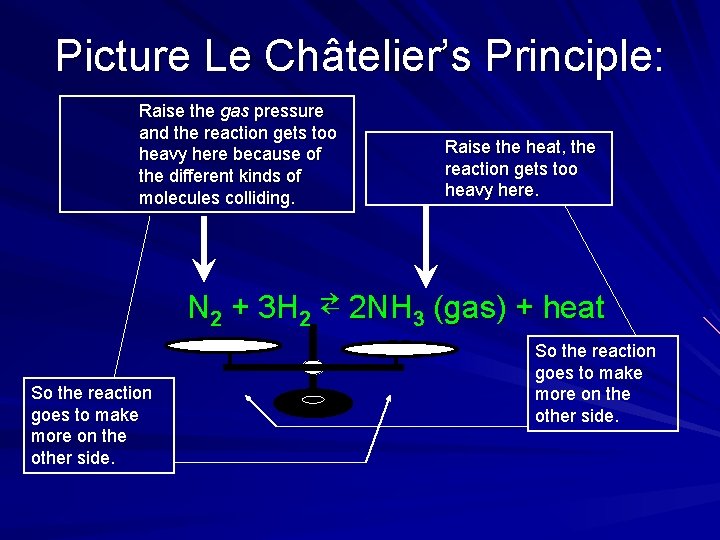
Standard Reactions & Reversible Reactions Some reactions go to completion and are done Others are reversible (⇄) – The reaction goes one way until it is unbalanced, then it goes the other way – Equilibrium = Dynamic balanced state Le Châtelier’s Principle: – Ex: N 2 + 3 H 2 ⇄ 2 NH 3 (gas) + heat ↑ heat and rxn moves to the left ↑gas pressure and rxn moves to the right (to the side with less separate molecules) Equilibrium is always sought by nature

Picture Le Châtelier’s Principle: Raise the gas pressure and the reaction gets too heavy here because of the different kinds of molecules colliding. Raise the heat, the reaction gets too heavy here. N 2 + 3 H 2 ⇄ 2 NH 3 (gas) + heat So the reaction goes to make more on the other side.