Chemical Reactions What is a chemical reaction A






- Slides: 14

Chemical Reactions

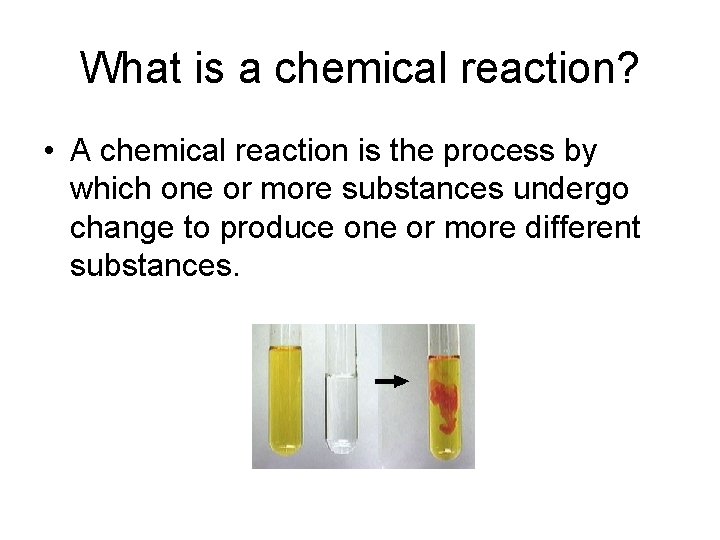
What is a chemical reaction? • A chemical reaction is the process by which one or more substances undergo change to produce one or more different substances.

5 signs a chemical reaction has taken place 1. Formation of gases or solids 2. Energy change (heat or electricity) 3. Color change 4. Odor change 5. Not easily reversed

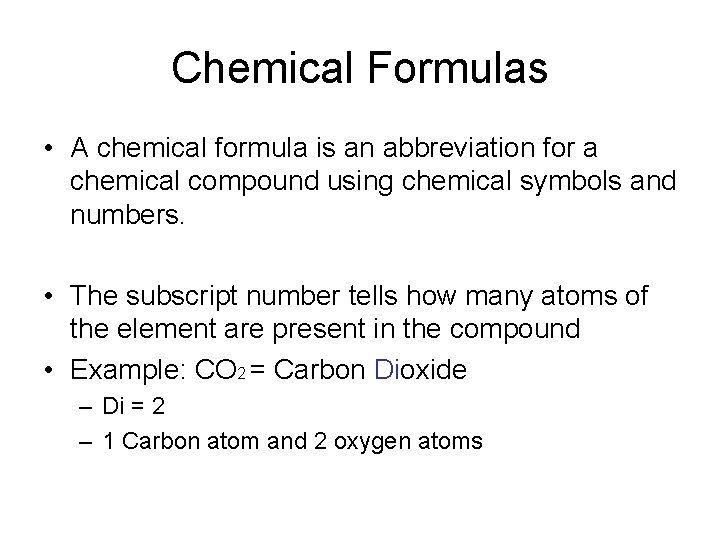
Chemical Formulas • A chemical formula is an abbreviation for a chemical compound using chemical symbols and numbers. • The subscript number tells how many atoms of the element are present in the compound • Example: CO 2 = Carbon Dioxide – Di = 2 – 1 Carbon atom and 2 oxygen atoms

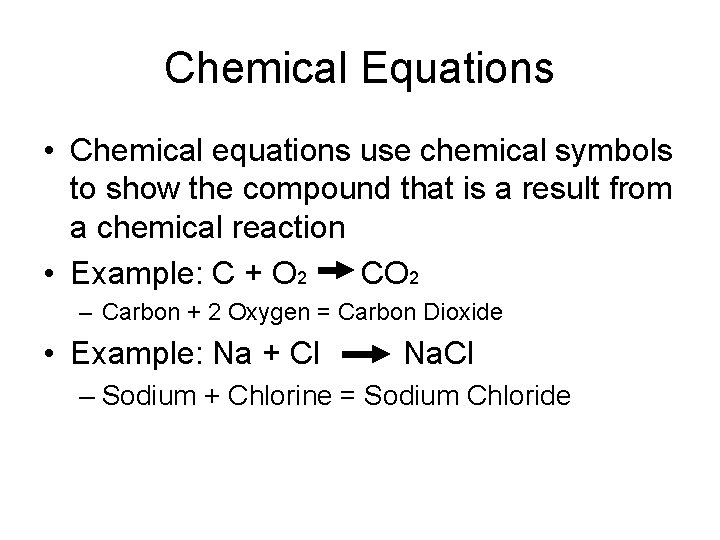
Chemical Equations • Chemical equations use chemical symbols to show the compound that is a result from a chemical reaction • Example: C + O 2 CO 2 – Carbon + 2 Oxygen = Carbon Dioxide • Example: Na + Cl Na. Cl – Sodium + Chlorine = Sodium Chloride

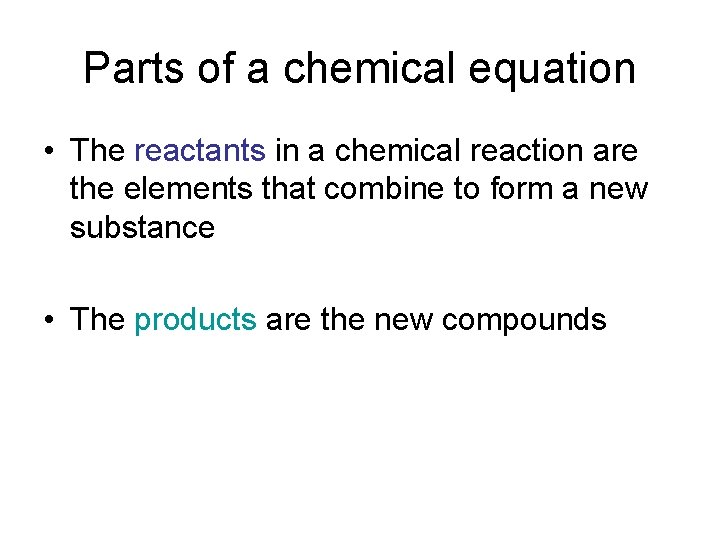
Parts of a chemical equation • The reactants in a chemical reaction are the elements that combine to form a new substance • The products are the new compounds

Rules for chemical equations • The total mass of the reactants must equal the total mass of the products • Equations must balance on both sides of the equation

Chemical Compounds • • • Ionic Covalent Acids Bases Salts

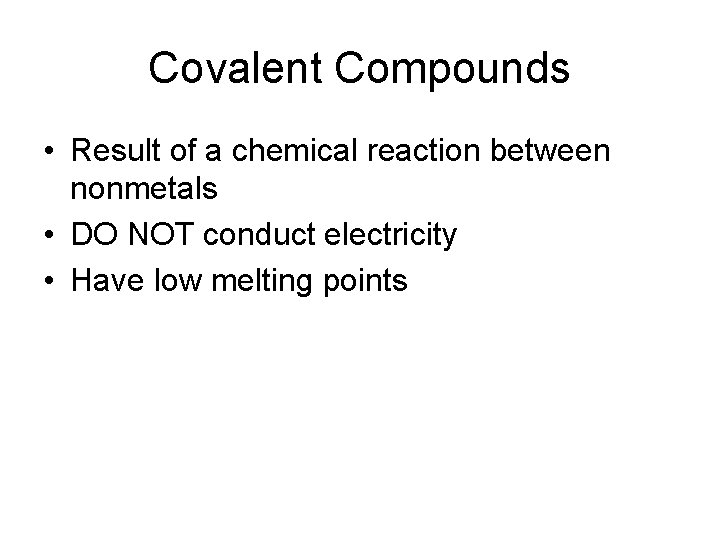
Covalent Compounds • Result of a chemical reaction between nonmetals • DO NOT conduct electricity • Have low melting points

Ionic Compounds • A chemical reaction formed between a metal and a nonmetal • Conduct electricity • Have high melting points • Brittle – ions shift when hit, this causes them to break apart

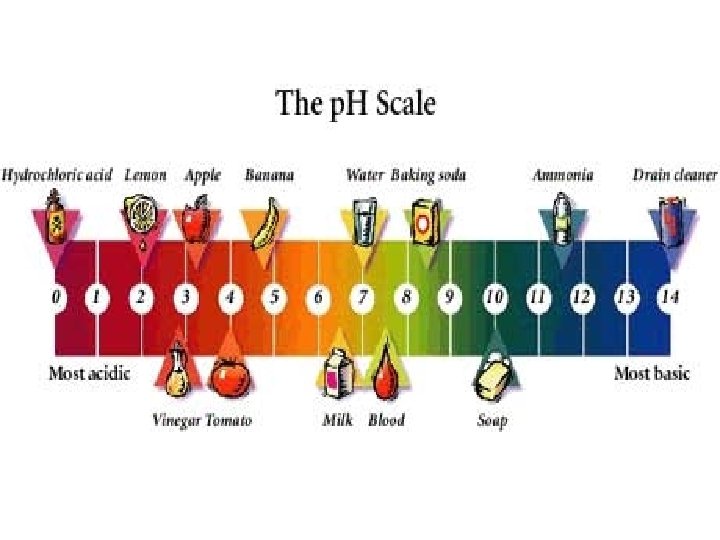
Acids • A compound that increases the number of hydrogen ions (H+) when dissolved in water • Acids react with metals producing hydrogen gas • Corrosive • Conduct electricity • Taste sour • Found in batteries, your stomach, soft drinks

Bases • Any compound that produces hydroxide ions (OH-) when dissolved in water • Feels slippery • Tastes bitter • Conduct electricity • Examples: soap, oven cleaners, drain uncloggers, other cleaning products, antacids


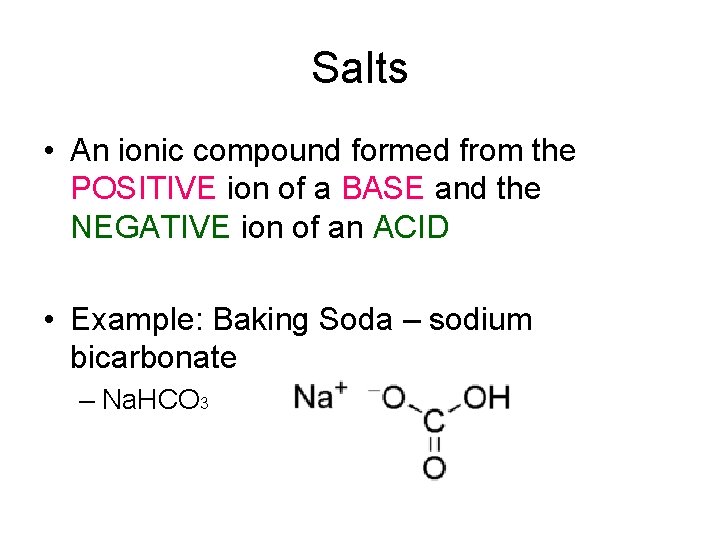
Salts • An ionic compound formed from the POSITIVE ion of a BASE and the NEGATIVE ion of an ACID • Example: Baking Soda – sodium bicarbonate – Na. HCO 3