Chemical Reactions What are chemical reactions There are
















- Slides: 22

Chemical Reactions

What are chemical reactions? • There are 6 major chemical reactions we need to study in this course • A chemical reaction has occurred when the starting chemical species (reactants) is different from the final chemical species (product) – Reactants → Products – The arrow shows the direction in which the reaction takes place, but the arrow does not explain time. The reaction can take a long time or short time.

Closed Systems • We want this system as it is our ideal system and we can do calculations • But very rare in nature • Must be done in very specific ideal conditions • Very expensive and hard to maintain • But open systems do not conserve anything, so we must always assume.

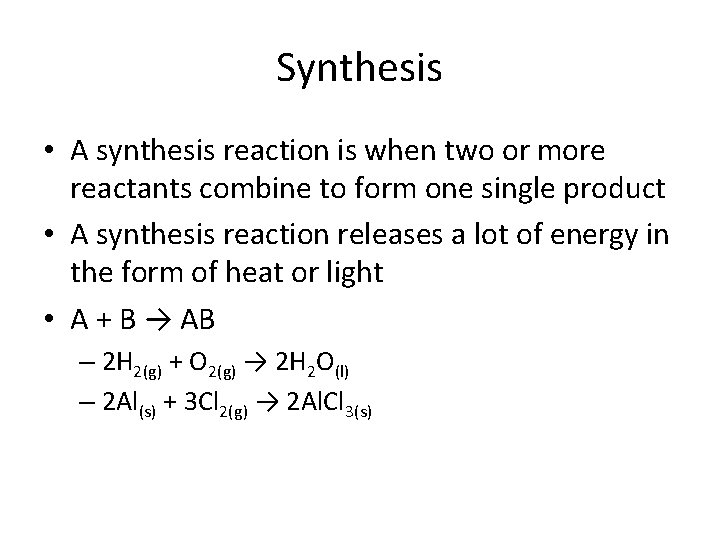
Synthesis • A synthesis reaction is when two or more reactants combine to form one single product • A synthesis reaction releases a lot of energy in the form of heat or light • A + B → AB – 2 H 2(g) + O 2(g) → 2 H 2 O(l) – 2 Al(s) + 3 Cl 2(g) → 2 Al. Cl 3(s)

Synthesis • What to look for? – We look for two elements reacting • How to predict the products? – We assume that the product is made up of the two elements reacting and joining together – So we write their product as if we are writing the formula for the chemical – Al + Cl 2 will be Al 3+ and Cl- so it will form Al. Cl 3 and then we balance.

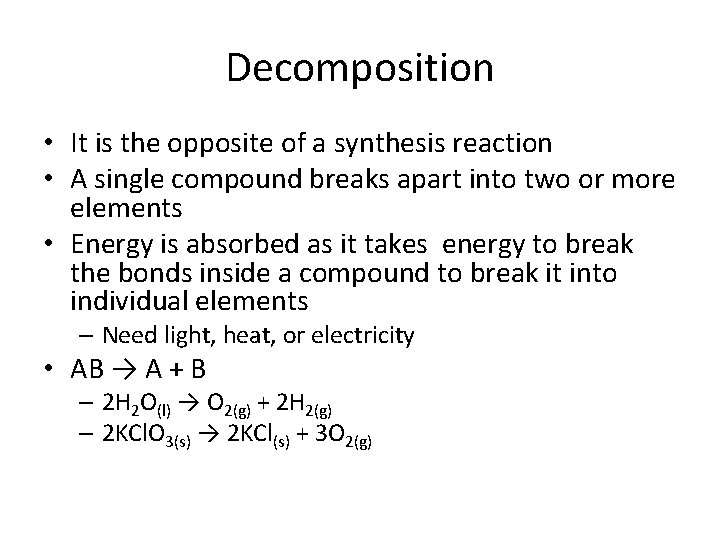
Decomposition • It is the opposite of a synthesis reaction • A single compound breaks apart into two or more elements • Energy is absorbed as it takes energy to break the bonds inside a compound to break it into individual elements – Need light, heat, or electricity • AB → A + B – 2 H 2 O(l) → O 2(g) + 2 H 2(g) – 2 KCl. O 3(s) → 2 KCl(s) + 3 O 2(g)

Decomposition • What to look for? – A single molecule on the reactant side • How to predict the products? – The single molecule will break into elements – So the product will be made up of the elements that the single molecule is made of – H 2 O is made up of H 2 and O 2, remember they are diatomics. – Fe. Cl 3 is made up of Fe and Cl 2

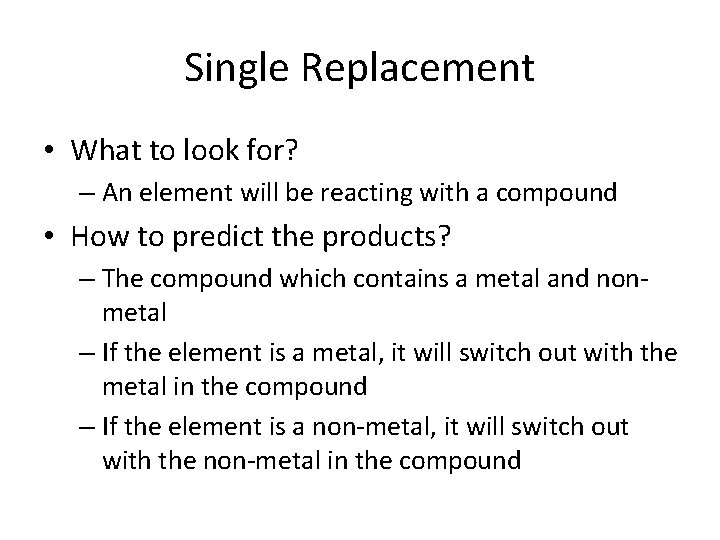
Single Replacement • A single replacement is when one element that is alone trades place with another element in a compound • A + BC → AC + B – Cu. Cl 2(s) + Fe(s) → Fe. Cl 2(s) + Cu(s) – Zn(s) + 2 HCl(aq) → Zn. Cl 2(g) + H 2(g) • This usually happens when a more reactive element kicks off the less reactive element that is in the compound • We will go more into this in the periodic trends

Single Replacement • What to look for? – An element will be reacting with a compound • How to predict the products? – The compound which contains a metal and nonmetal – If the element is a metal, it will switch out with the metal in the compound – If the element is a non-metal, it will switch out with the non-metal in the compound

Double Replacement • This happens between two ionic compounds • It is when the metal (cation) and the nonmetal (anion) switch partners • AB + CD → AD + CB – 2 Na. Cl(s) + H 2 SO 4(aq) → 2 HCl(aq) + Na 2 SO 4(s)

Double Replacement • What to look for? – Two ionic compound • How to predict the products? – The products will be the swapping of partners of the metal and non-metal • Usually, this reaction takes place to form water or to form an insoluble compound (solid precipitate). – You will learn more in Chemistry 12.

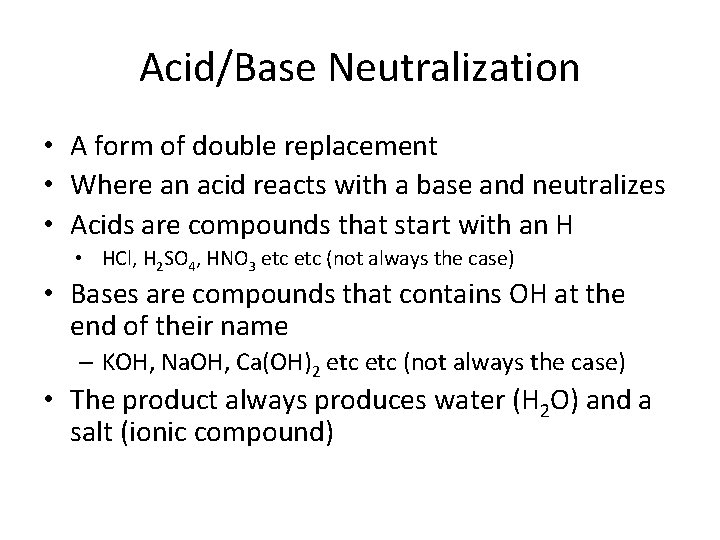
Acid/Base Neutralization • A form of double replacement • Where an acid reacts with a base and neutralizes • Acids are compounds that start with an H • HCl, H 2 SO 4, HNO 3 etc (not always the case) • Bases are compounds that contains OH at the end of their name – KOH, Na. OH, Ca(OH)2 etc (not always the case) • The product always produces water (H 2 O) and a salt (ionic compound)

Acid/Base Neutralization • HA + BOH → HOH + BA – HCl(aq) + Na. OH(aq) → HOH(l) + Na. Cl(s/aq) – 2 KOH(aq) + H 2 SO 4(aq) → 2 H 2 O(l) + K 2 SO 4(aq) – HOH = H 2 O • A salt is any ionic compound that is neutral and doesn’t contain H at the start of their name or OH at the end of their name

Acid/Base Neutralization • What to look for? – An acid and base reacting • How to predict the products? – The products will always form water (H 2 O) and the salt will be the metal and non-metal left over.

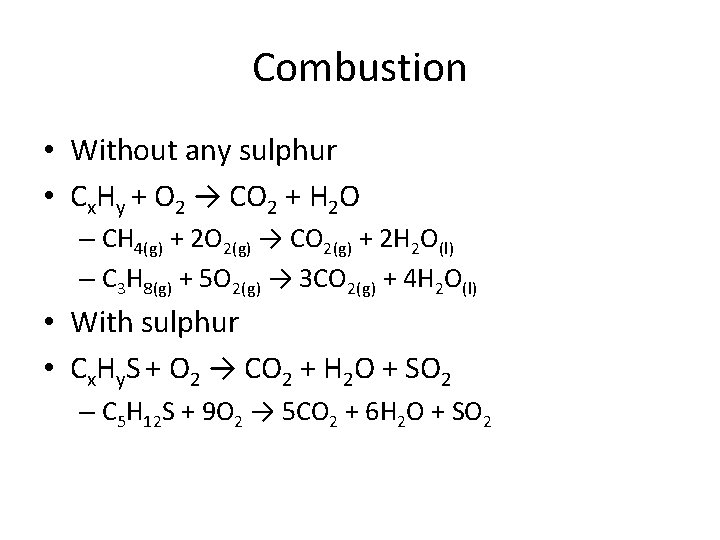
Combustion • A combustion reaction is a reaction that is between a hydrocarbon (carbon and hydrogen based substance) and oxygen gas (O 2) – We know it as either a fire or an explosion • The product will always produce CO 2 and H 2 O – If the reactant has sulphur, it will produce SO 2 as an additional product • It also releases energy in the form of heat or light

Combustion • Without any sulphur • Cx. Hy + O 2 → CO 2 + H 2 O – CH 4(g) + 2 O 2(g) → CO 2(g) + 2 H 2 O(l) – C 3 H 8(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O(l) • With sulphur • Cx. Hy. S + O 2 → CO 2 + H 2 O + SO 2 – C 5 H 12 S + 9 O 2 → 5 CO 2 + 6 H 2 O + SO 2

Combustion • What to look for? – A hydrocarbon reacting with oxygen gas • How to predict the products? – If the reactant contains no sulphur, the product is CO 2 and H 2 O – If the reactant contains sulphur, add SO 2 to the products

Note! • When SO 2 is formed, it will react with water in our atmosphere. • When this happens, it produces sulphurous acid H 2 SO 3 or acid rain • SO 2 is produced by our cars • It can choke you if you breathe it in as it replaces the oxygen in your lungs (happened before)

Page 118 for summary table of chemical reactions

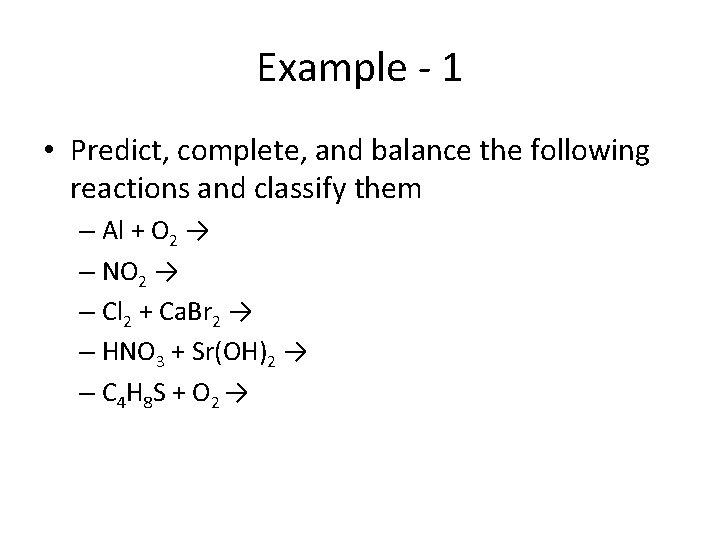
Example - 1 • Predict, complete, and balance the following reactions and classify them – Al + O 2 → – NO 2 → – Cl 2 + Ca. Br 2 → – HNO 3 + Sr(OH)2 → – C 4 H 8 S + O 2 →

Video https: //www. youtube. com/watch? v= ns. Ek. KIi. Oz 7 Q

Practice • Page 118 - #65 -67
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Mikael ferm
Mikael ferm Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Section 1 chemical changes
Section 1 chemical changes Are kc and kp equal
Are kc and kp equal Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Redox reaction
Redox reaction 4 types of chemical reactions
4 types of chemical reactions Three types of chemical reactions
Three types of chemical reactions Chemical reactions in soil
Chemical reactions in soil 5 chemical reactions
5 chemical reactions Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Predicting products of chemical reactions
Predicting products of chemical reactions Chapter 9 chemical reactions
Chapter 9 chemical reactions Combustion chemical reaction
Combustion chemical reaction Indications of chemical reactions
Indications of chemical reactions Www.biology-roots.com
Www.biology-roots.com Rules for chemical reactions
Rules for chemical reactions Jared investigated chemical reactions
Jared investigated chemical reactions Ouchterlony
Ouchterlony Chemical reaction in bread
Chemical reaction in bread