Chemical Reactions There are five types of chemical

- Slides: 12

Chemical Reactions

There are five types of chemical reactions Type of reaction Single replacement Double replacement Synthesis Decomposition Combustion Definition Example

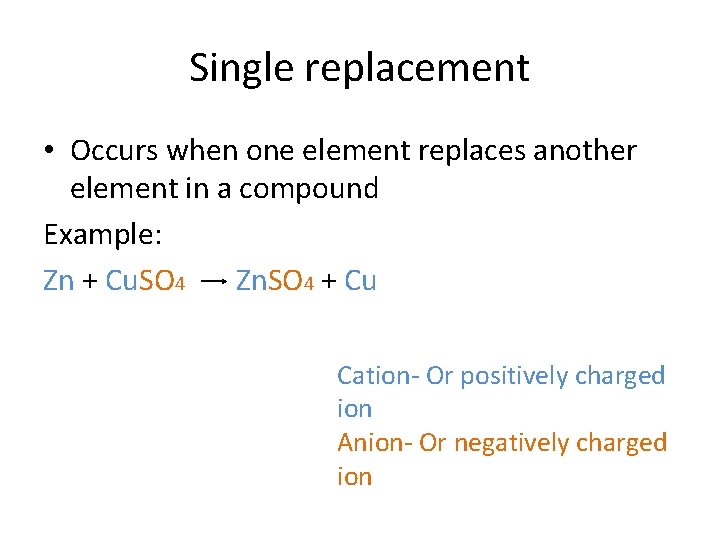
Single replacement • Occurs when one element replaces another element in a compound Example: Zn + Cu. SO 4 Zn. SO 4 + Cu Cation- Or positively charged ion Anion- Or negatively charged ion

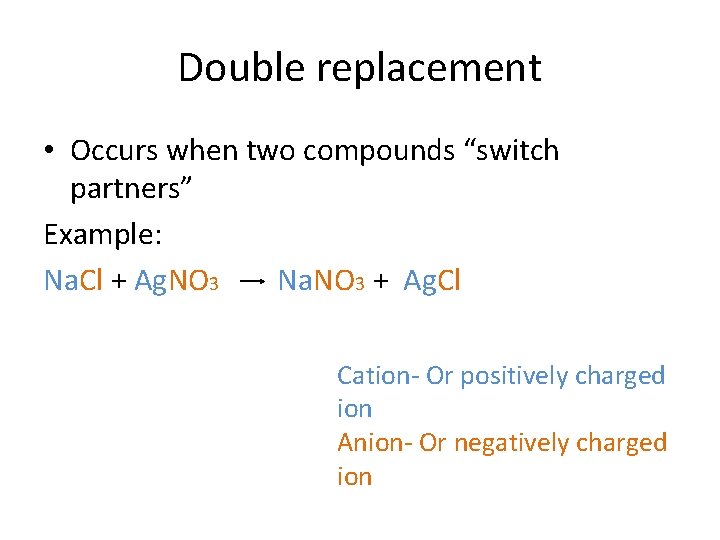
Double replacement • Occurs when two compounds “switch partners” Example: Na. Cl + Ag. NO 3 Na. NO 3 + Ag. Cl Cation- Or positively charged ion Anion- Or negatively charged ion

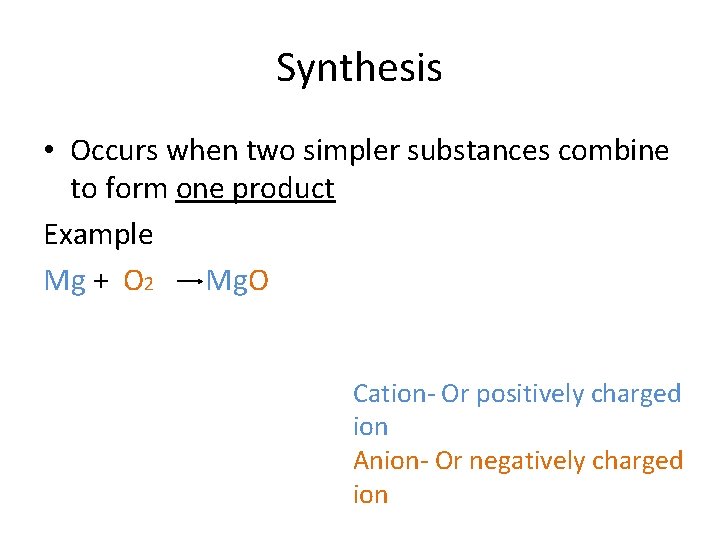
Synthesis • Occurs when two simpler substances combine to form one product Example Mg + O 2 Mg. O Cation- Or positively charged ion Anion- Or negatively charged ion

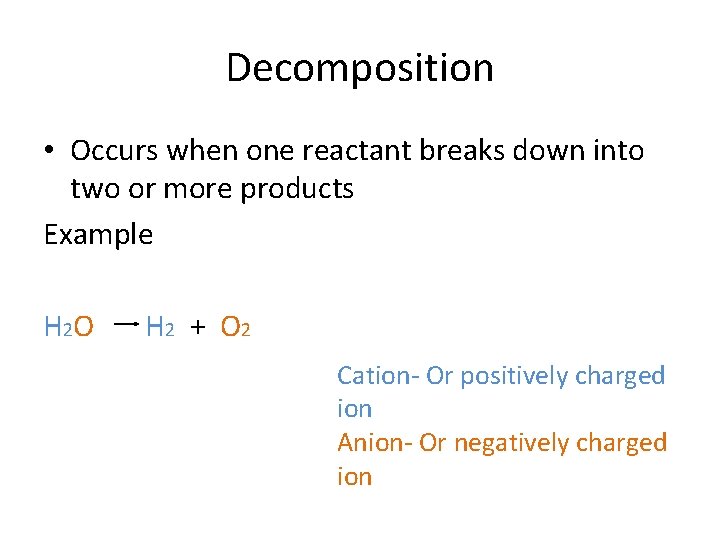
Decomposition • Occurs when one reactant breaks down into two or more products Example H 2 O H 2 + O 2 Cation- Or positively charged ion Anion- Or negatively charged ion

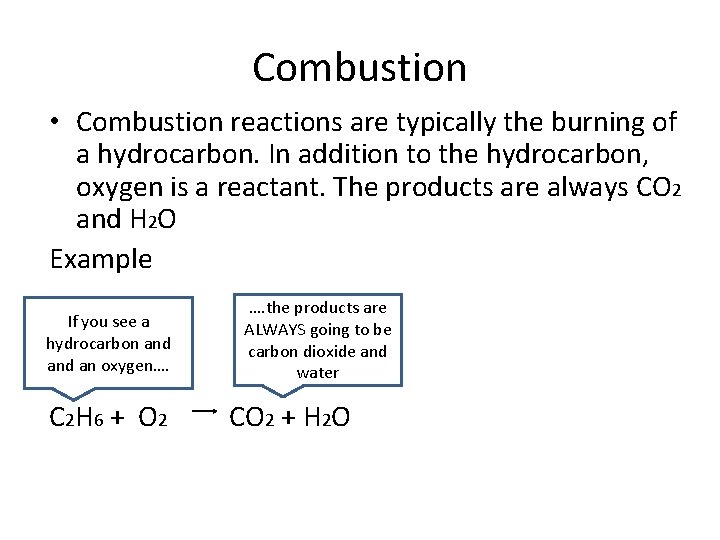
Combustion • Combustion reactions are typically the burning of a hydrocarbon. In addition to the hydrocarbon, oxygen is a reactant. The products are always CO 2 and H 2 O Example If you see a hydrocarbon and an oxygen…. C 2 H 6 + O 2 …. the products are ALWAYS going to be carbon dioxide and water CO 2 + H 2 O

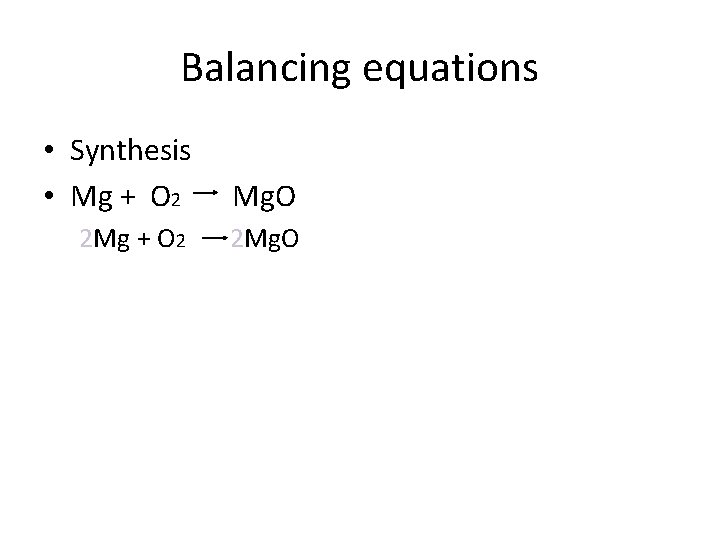
Balancing equations • Synthesis • Mg + O 2 Mg. O 2 Mg + O 2 2 Mg. O

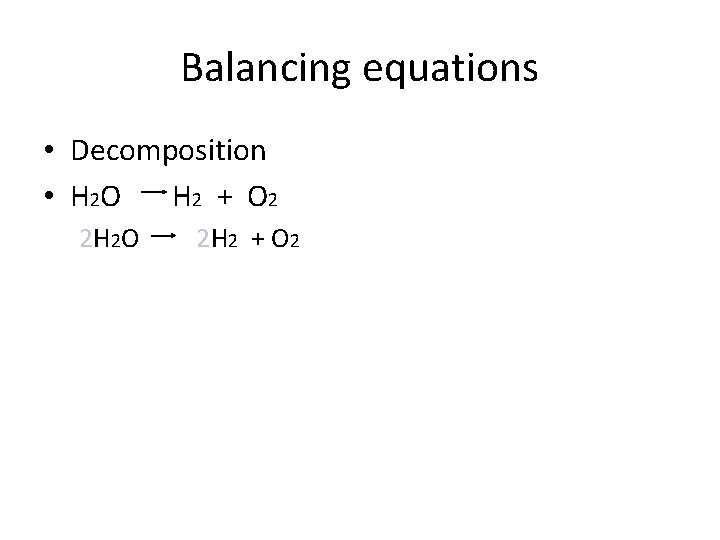
Balancing equations • Decomposition • H 2 O H 2 + O 2 2 H 2 O 2 H 2 + O 2

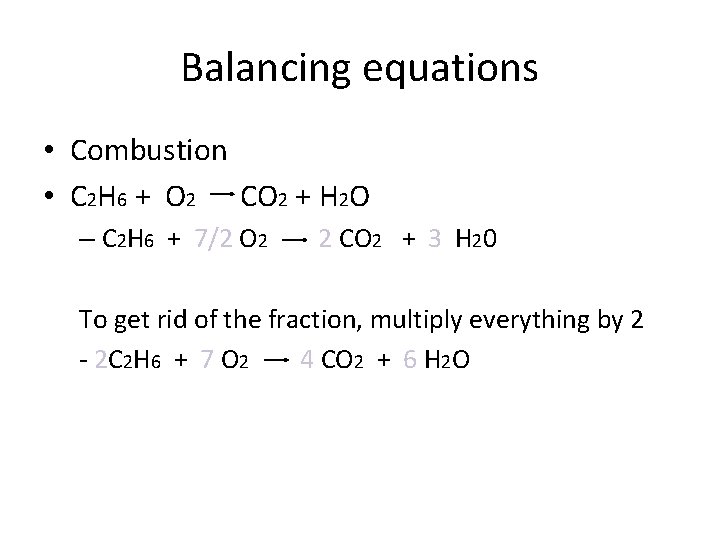
Balancing equations • Combustion • C 2 H 6 + O 2 CO 2 + H 2 O – C 2 H 6 + 7/2 O 2 2 CO 2 + 3 H 20 To get rid of the fraction, multiply everything by 2 - 2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O

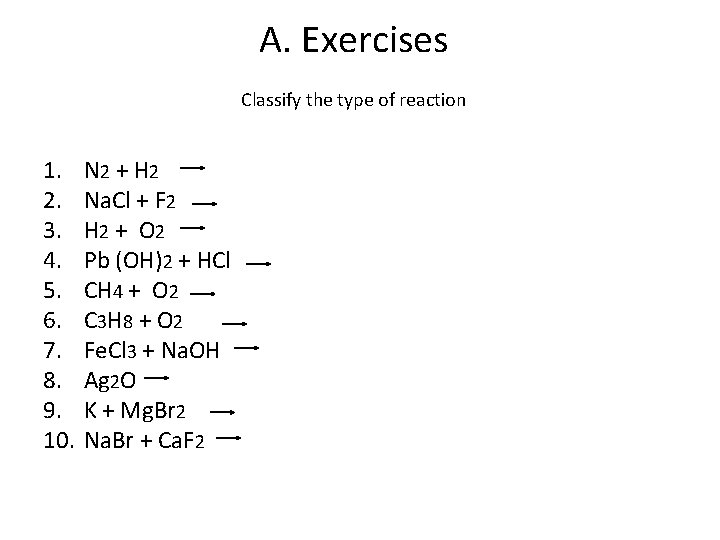
A. Exercises Classify the type of reaction 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. N 2 + H 2 Na. Cl + F 2 H 2 + O 2 Pb (OH)2 + HCl CH 4 + O 2 C 3 H 8 + O 2 Fe. Cl 3 + Na. OH Ag 2 O K + Mg. Br 2 Na. Br + Ca. F 2

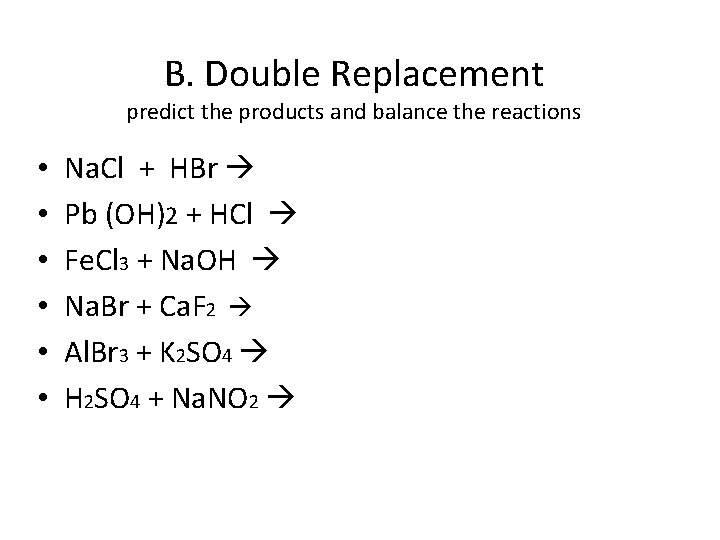
B. Double Replacement predict the products and balance the reactions • • • Na. Cl + HBr Pb (OH)2 + HCl Fe. Cl 3 + Na. OH Na. Br + Ca. F 2 Al. Br 3 + K 2 SO 4 H 2 SO 4 + Na. NO 2