CHEMICAL REACTIONS The Atom Electron shell Negatively charged










- Slides: 32

CHEMICAL REACTIONS

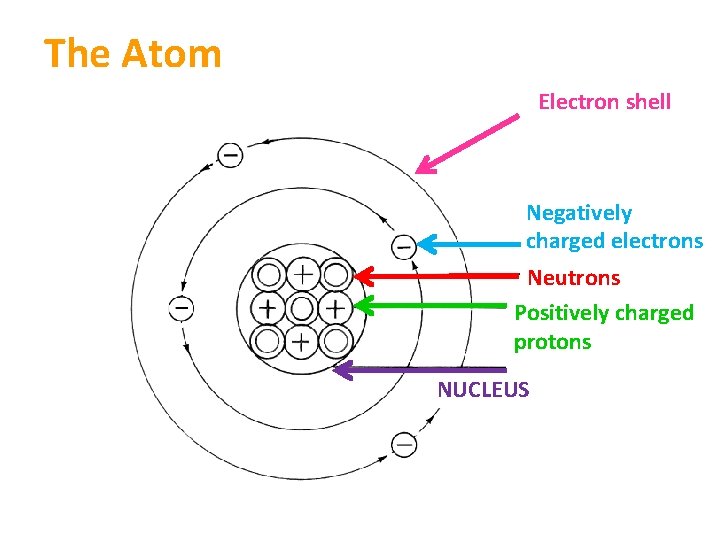
The Atom Electron shell Negatively charged electrons Neutrons Positively charged protons NUCLEUS

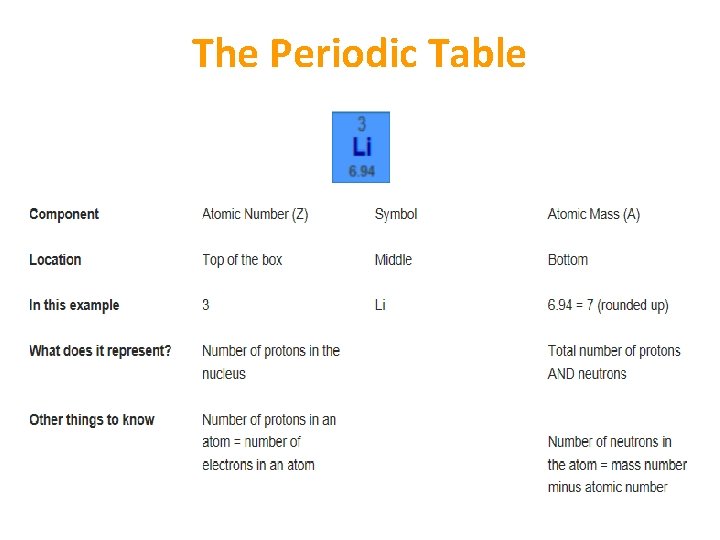
The Atom • An atom has a neutral charge: number of protons = number of electrons • 2 electrons can fit in the first shell of an atom & 8 in each subsequent shell • Atomic number (Z) = number of protons in an atom’s nucleus • Mass number (A) = number of protons + number of neutrons • Number of neutrons in an atom = mass number minus atomic number

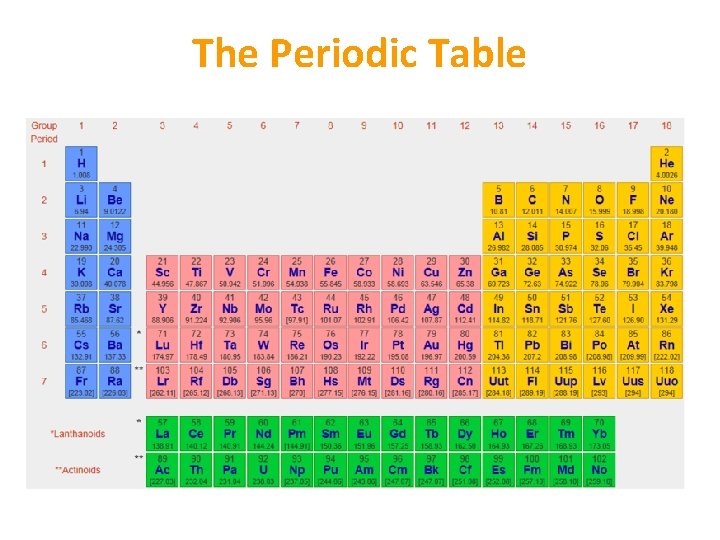
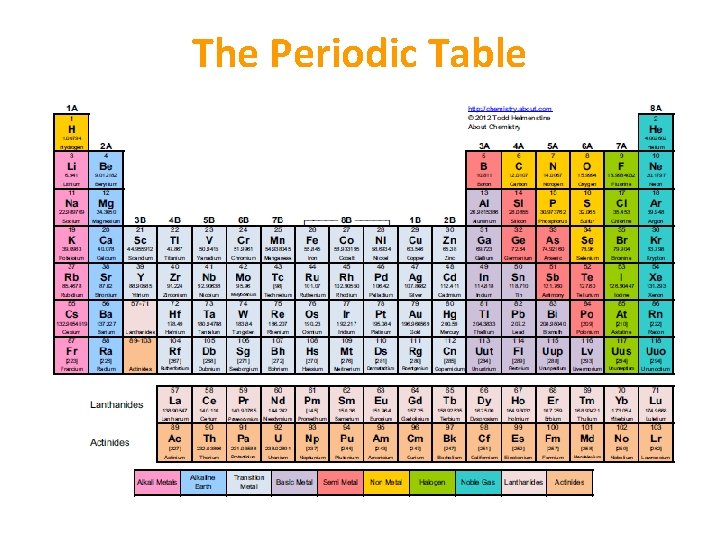
The Periodic Table • Table created to group elements according to their properties • Vertical columns = groups • Horizontal rows = periods • Group number = number of electrons in outer shell • Period number = number of shells

The Periodic Table

The Periodic Table

The Periodic Table

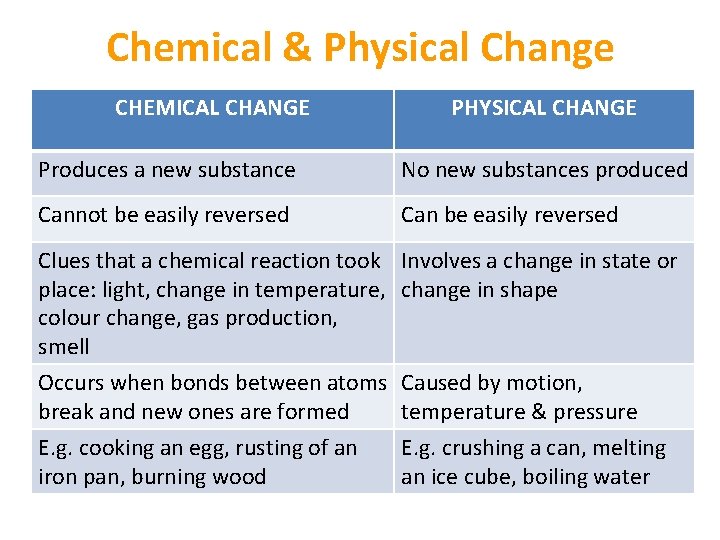
Chemical & Physical Change CHEMICAL CHANGE PHYSICAL CHANGE Produces a new substance No new substances produced Cannot be easily reversed Can be easily reversed Clues that a chemical reaction took place: light, change in temperature, colour change, gas production, smell Occurs when bonds between atoms break and new ones are formed E. g. cooking an egg, rusting of an iron pan, burning wood Involves a change in state or change in shape Caused by motion, temperature & pressure E. g. crushing a can, melting an ice cube, boiling water

Chemical OR Physical? • Burning paper CHEMICAL • Baking a cake CHEMICAL • Sweat cools you as it evaporates PHYSICAL • Boiling water added to instant coffee • Photosynthesis CHEMICAL • Margarine is melted PHYSICAL

Chemical Reactions • Occur when bonds between atoms break and new ones are formed • Reactants Products

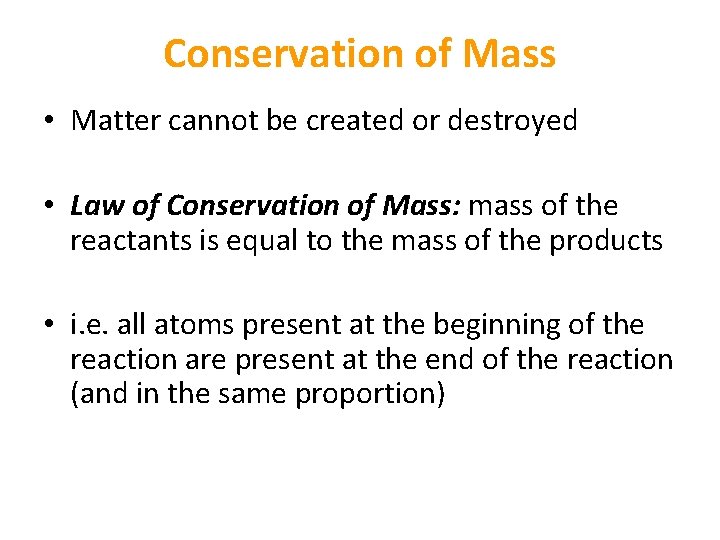
Conservation of Mass • Matter cannot be created or destroyed • Law of Conservation of Mass: mass of the reactants is equal to the mass of the products • i. e. all atoms present at the beginning of the reaction are present at the end of the reaction (and in the same proportion)

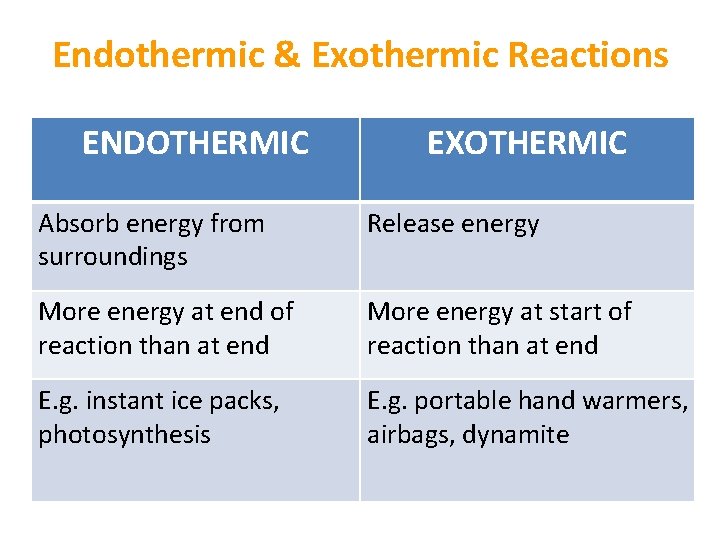
Endothermic & Exothermic Reactions ENDOTHERMIC EXOTHERMIC Absorb energy from surroundings Release energy More energy at end of reaction than at end More energy at start of reaction than at end E. g. instant ice packs, photosynthesis E. g. portable hand warmers, airbags, dynamite

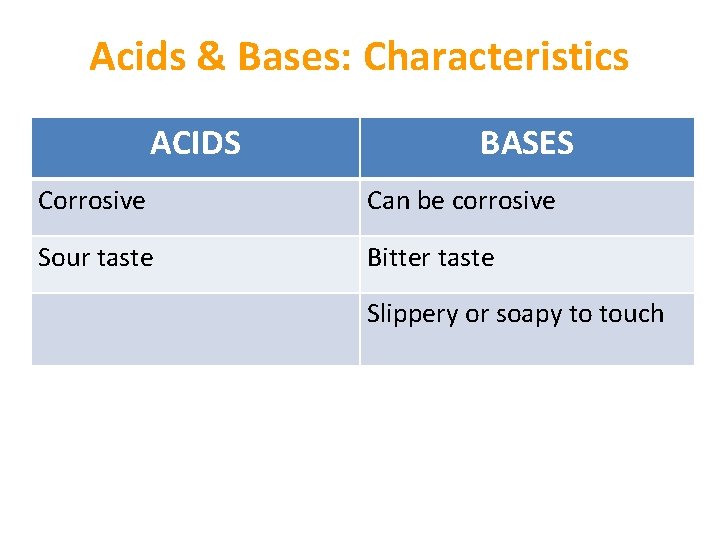
Acids & Bases: Characteristics ACIDS BASES Corrosive Can be corrosive Sour taste Bitter taste Slippery or soapy to touch

Acids or Bases?

Acid-Base Indicators • Added to a substance to determine if it is an acid or a base • Litmus: – Turns RED in an acid and BLUE in a base • Bromothymol blue: – Turns YELLOW in an acid and BLUISH- PURPLE in a base

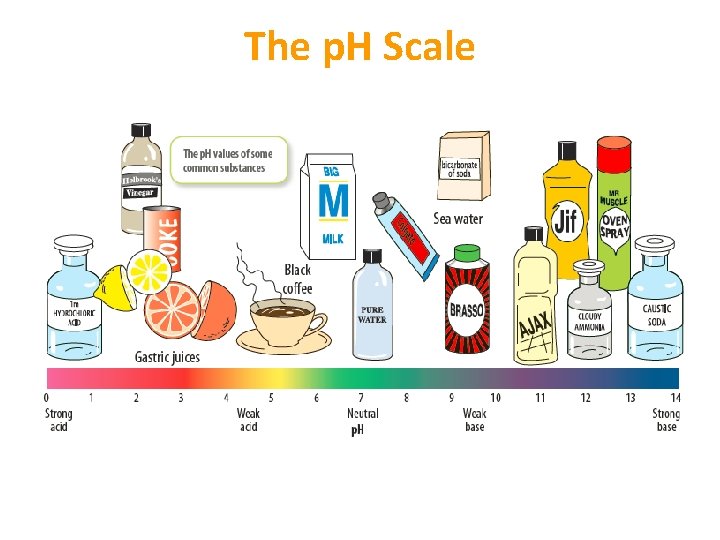
The p. H Scale • Shows the relative strength of an acid or a base • p. H of a substance can be determined using a universal indicator • Is a mixture of indicators that will change colour based on the strength of the acid or the base

The p. H Scale

Neutralisation • Acid + base water + a salt – Sometimes a gas is also a product • Stop the effects of an acid by adding a base • Stop the effects of a base by adding an acid • Examples: – Relieve an ant sting by adding a weak base (e. g. sodium bicarbonate – baking soda) – Relieve a wasp sting by adding a weak acid (e. g. vinegar)

Neutralisation Examples • Certain plants grow better in acidic/basic soils • Add lime if soil too acidic • Add ammonium sulfate if soil is too basic

Neutralisation Examples • p. H of a swimming pool should be between 7. 2 – 7. 8 • If above 7. 8, allows bacteria & microorganisms to grow need to add an acid (e. g. sodium hydroxide) • If below 7. 2, swimmers will get red & stinging eyes & the water may become corrosive need to add a base (e. g. sodium bicarbonate)

Neutralisation Examples • If contents of stomach become too acidic (by eating too quickly or having too much of the wrong food) you will feel a burning sensation • Can be relieved by taking an antacid, which contain weak bases (e. g. aluminium hydroxide)

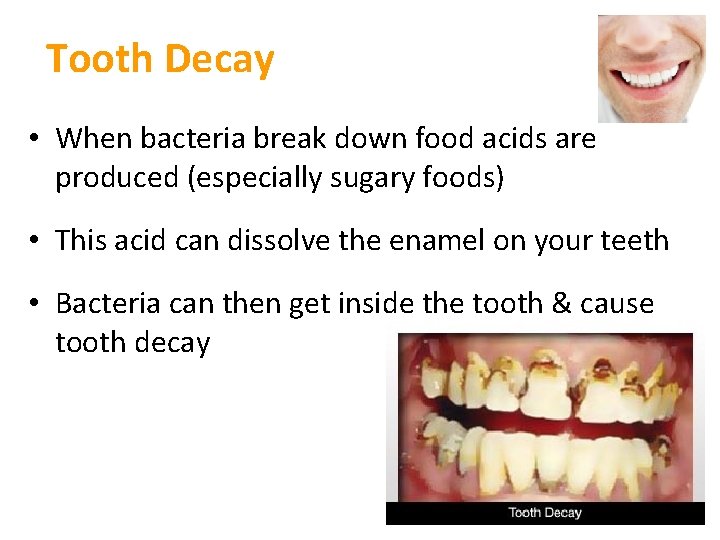
Tooth Decay • When bacteria break down food acids are produced (especially sugary foods) • This acid can dissolve the enamel on your teeth • Bacteria can then get inside the tooth & cause tooth decay

Acids & Metals • Acid + metal a salt + hydrogen • For example: – Sulfuric acid + copper sulfate + hydrogen

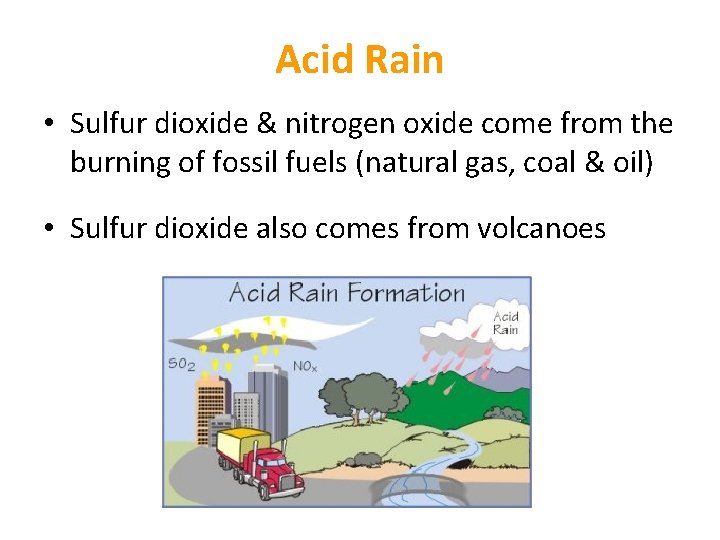
Acid Rain • As rain falls, water reacts with carbon dioxide in the air • This forms a weak acid • However, if there is a lot of sulfur dioxide and nitrogen oxide in the atmosphere, water reacts with these gases • This produces sulfuric acid & nitric acid rain is more acidic than normal

Acid Rain • Sulfur dioxide & nitrogen oxide come from the burning of fossil fuels (natural gas, coal & oil) • Sulfur dioxide also comes from volcanoes

Acid Rain: Problems • Eats into buildings & statues • Damages cells on leaves & affects water flow through plants • Makes plants more likely to be damaged by frost, fungi & disease

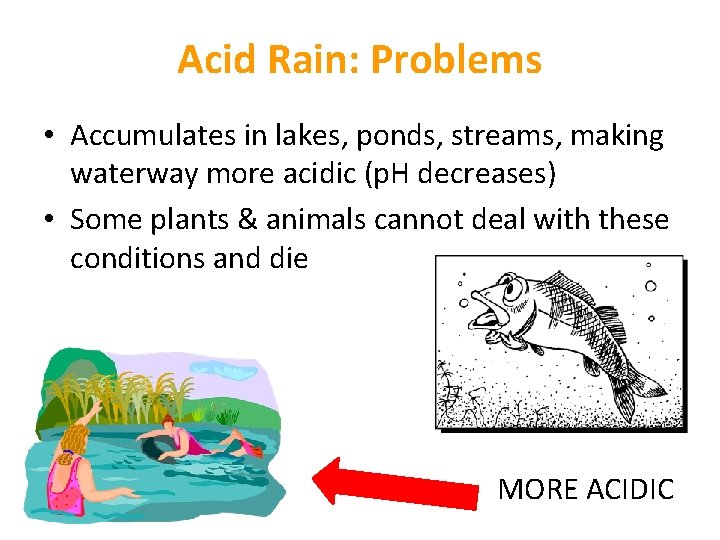
Acid Rain: Problems • Accumulates in lakes, ponds, streams, making waterway more acidic (p. H decreases) • Some plants & animals cannot deal with these conditions and die MORE ACIDIC

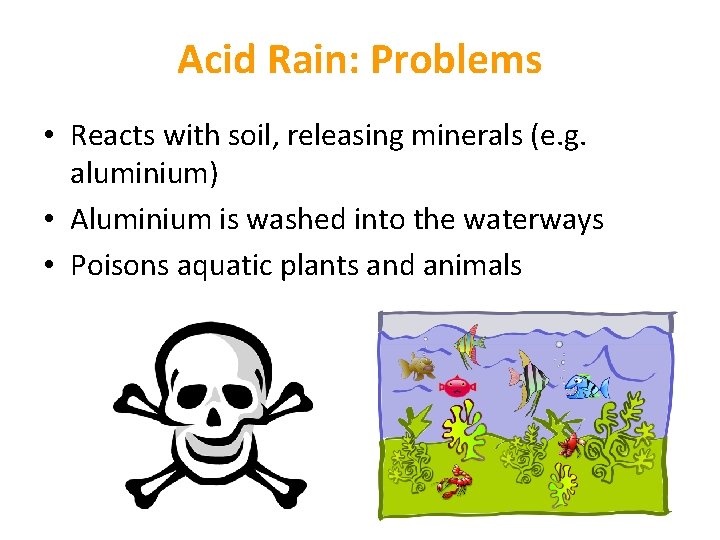
Acid Rain: Problems • Reacts with soil, releasing minerals (e. g. aluminium) • Aluminium is washed into the waterways • Poisons aquatic plants and animals

Acid Rain: Solutions • Alternative ways of producing electricity • Using public transport or car pooling

Types of Chemical Reactions: Combustion • Combustion reaction: a substance (called a fuel) reacts with oxygen and heat is produced • Fuel + oxygen carbon dioxide + water • Examples of fuels: natural gas, petrol, coal • Combustion can also produce dangerous gases (e. g. carbon monoxide)

Combustion Examples • Gas stoves – Natural gas (methane) + oxygen carbon dioxide + water • Cars – Fuel (octane) + oxygen carbon dioxide + water • Aeroplanes – Fuel (kerosene) + oxygen carbon dioxide + water • Electricity – Coal + oxygen carbon dioxide + water

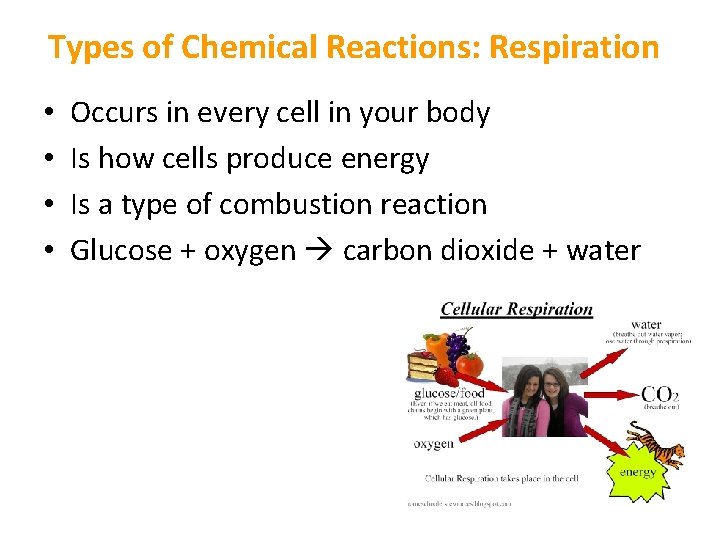
Types of Chemical Reactions: Respiration • • Occurs in every cell in your body Is how cells produce energy Is a type of combustion reaction Glucose + oxygen carbon dioxide + water