Chemical Reactions The 5 Basic Classifications SYNTHESIS A

Chemical Reactions The 5 Basic Classifications

SYNTHESIS • A reaction in which two or more reactants yield a single product. • Also called composition or combination • General Equation A + B AB • EX: 2 Li + Se ---> Li 2 Se

Decomposition Reaction • One Reactant Breaking Down into two or more products • General Equation • AB ---> A + B • Example: • 2 Hg. O 2 Hg + O 2
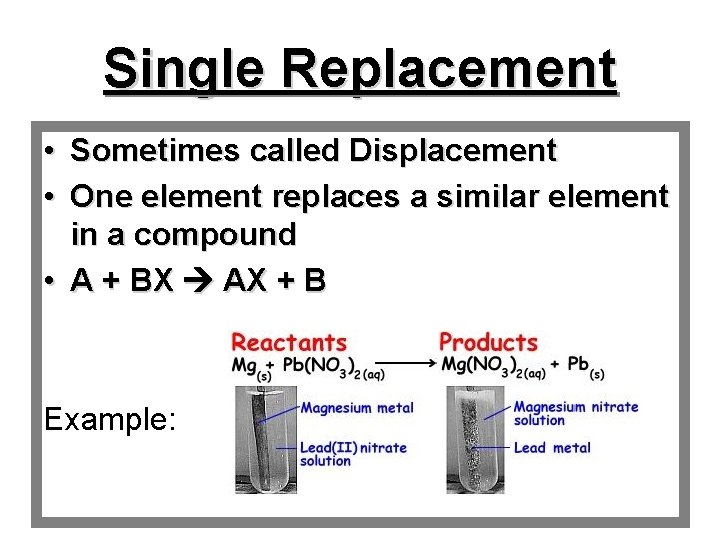
Single Replacement • Sometimes called Displacement • One element replaces a similar element in a compound • A + BX AX + B Example:

Double Replacement • Reaction that has the interchanging of two ions from two different compounds. • general form: AB + CD----> AD + CB • Example: Pb(NO 3)2 + 2 KI ----> Pb. I 2 + 2 KNO 3

Double Replacement • Equation consists of two reactants that have both a cation and anion. • During a reaction the cations (or anions) switch places. • The products usually consist of a precipitate.

What Is Combustion? One or more reactants combine with oxygen releasing heat or light Any combustion reaction must include the reactant oxygen, O 2 General Equation: A + O 2 AO • Example: • 2 Mg(s) + O 2(g) 2 Mg. O(s)

Reaction Checklist • 1) One product? (synthesis) • 2) One reactant? (decomposition) • 3) Is an element being replaced? (single) • 4) 2 switches? (double) • 5) Is O 2 a reactant? (combustion)
- Slides: 8