Chemical Reactions Section 9 1 Reactions and Equations




































- Slides: 81


Chemical Reactions Section 9. 1 Reactions and Equations Section 9. 2 Classifying Chemical Reactions Section 9. 3 Reactions in Aqueous Solutions Click a hyperlink or folder tab to view the corresponding slides. Exit

Section 9. 1 Reactions and Equations • Recognize evidence of chemical change. • Represent chemical reactions with equations. • Balance chemical equations. chemical change: a process involving one or more substances changing into a new substance chemical reaction reactant Chemical reactions are represented by balanced chemical equations. product chemical equation coefficient

Chemical Reactions • The process by which one or more substances are rearranged to form different substances is called a chemical reaction. • Another name for a chemical reaction is a chemical change.

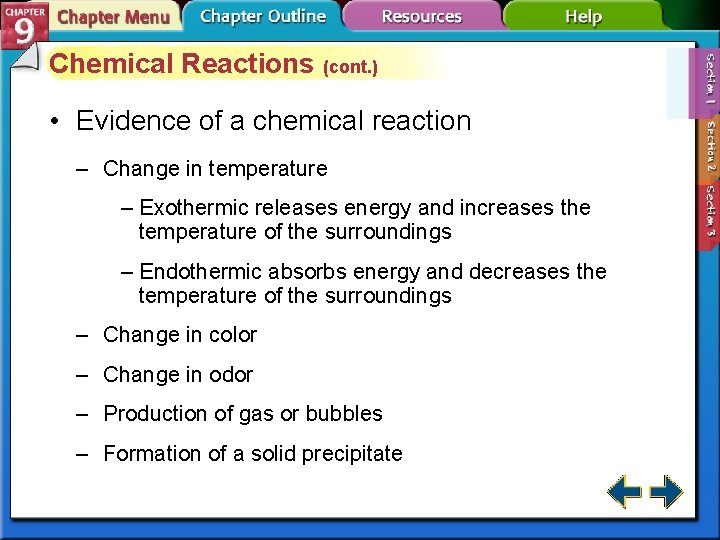
Chemical Reactions (cont. ) • Evidence of a chemical reaction – Change in temperature – Exothermic releases energy and increases the temperature of the surroundings – Endothermic absorbs energy and decreases the temperature of the surroundings – Change in color – Change in odor – Production of gas or bubbles – Formation of a solid precipitate

Representing Chemical Reactions • Chemists use statements called equations to represent chemical reactions. • Reactants are the starting substances. • Products are the substances formed in the reaction.

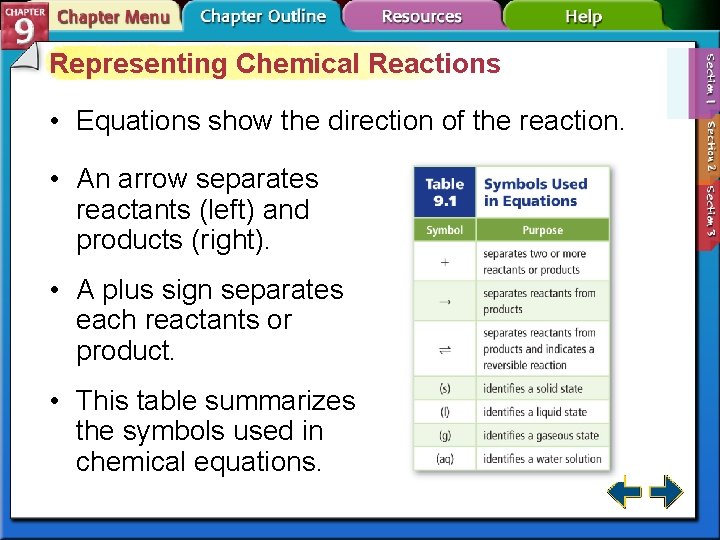
Representing Chemical Reactions • Equations show the direction of the reaction. • An arrow separates reactants (left) and products (right). • A plus sign separates each reactants or product. • This table summarizes the symbols used in chemical equations.

Representing Chemical Reactions (cont. ) • In word equations, aluminum(s) + bromine(l) → aluminum bromide(s) reads as “aluminum and bromine react to produce aluminum bromide”. • Skeleton equations use symbols/formulas to represent the reactants and products. Al(s) + Br(l) → Al. Br 3(s) • Skeleton equations lack information about how many atoms are involved in the reaction.

• Skeleton and word equations lack information about reactions. • The law of conservation states that matter is neither created nor destroyed in a chemical reaction. • Thus, chemical equations must show that matter is conserved. • The number of atoms of each reactant or product must be equal on both sides of the equation. • Such an equation is called a balanced chemical equation.

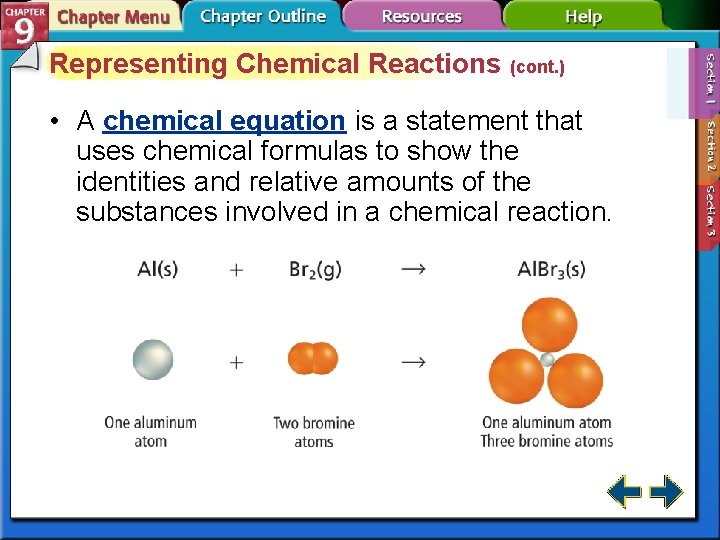
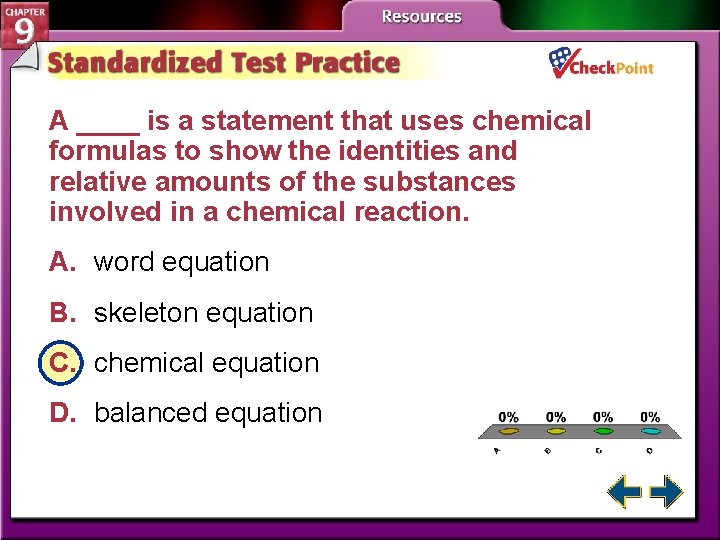
Representing Chemical Reactions (cont. ) • A chemical equation is a statement that uses chemical formulas to show the identities and relative amounts of the substances involved in a chemical reaction.

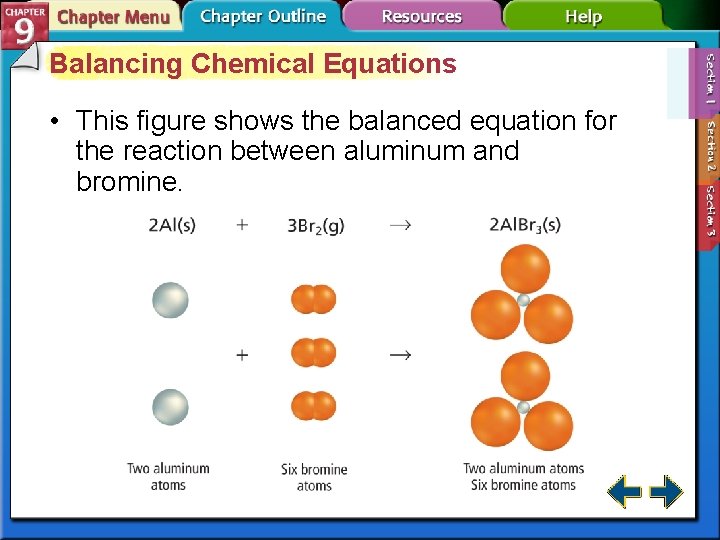
Balancing Chemical Equations • This figure shows the balanced equation for the reaction between aluminum and bromine.

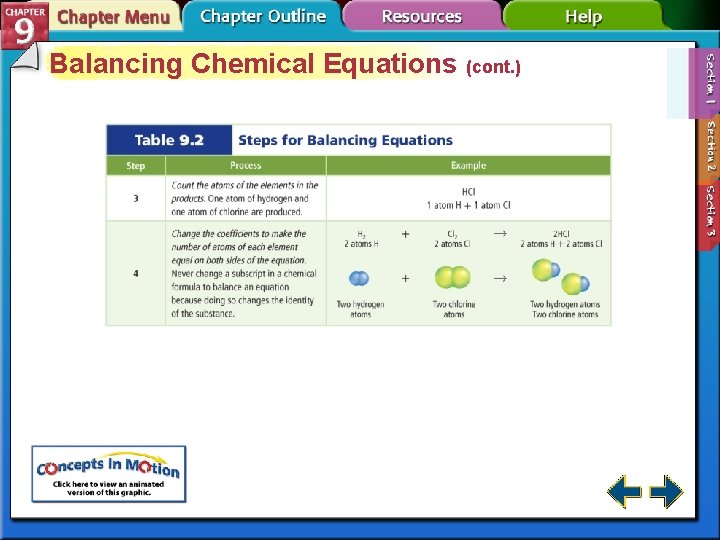
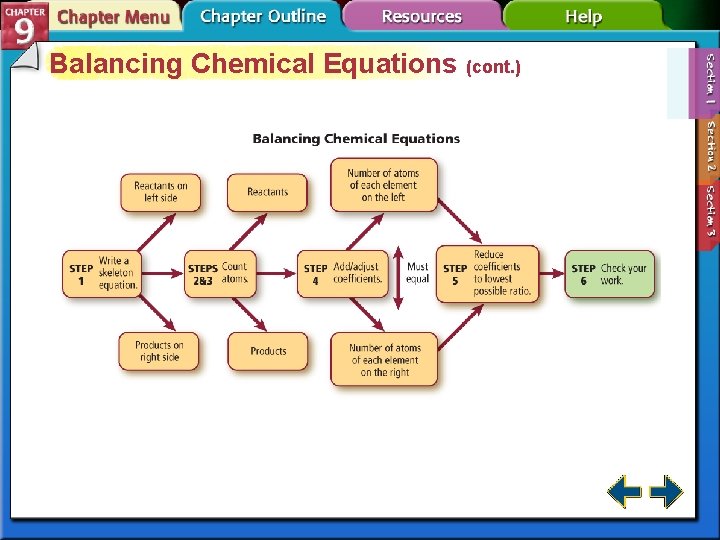
Balancing Chemical Equations (cont. ) • To balance a chemical equation, the correct coefficients must be added to the skeletal equation. • A coefficient in a chemical equation is a whole-number written in front of a reactant or product, describing the lowest whole-number ratio of the amounts of all the reactants and products. • It is not necessary to write the coefficient of 1. It is considered to be understood.

Balancing Chemical Equations (cont. )

Balancing Chemical Equations (cont. )

Balancing Chemical Equations (cont. )

Balancing Chemical Equations (cont. )

Section 9. 1 Assessment Which of the following is NOT a chemical reaction? A. a piece of wood burning B. a car rusting C. an ice cube melting into water D. red litmus paper turning blue A. B. C. D. A B C D

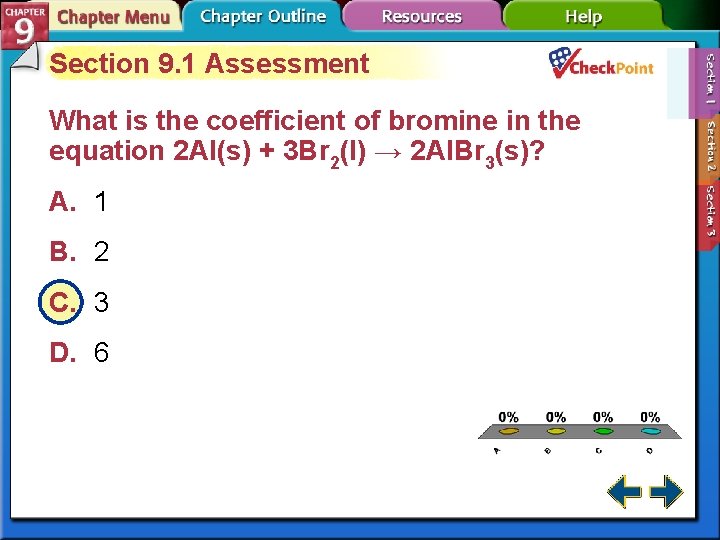
Section 9. 1 Assessment What is the coefficient of bromine in the equation 2 Al(s) + 3 Br 2(l) → 2 Al. Br 3(s)? A. 1 B. 2 C. 3 D. 6 A. B. C. D. A B C D


Section 9. 2 Classifying Chemical Reactions • Classify chemical reactions. • Identify the characteristics of different classes of chemical reactions. metal: an element that is a solid at room temperature, a good conductor of heat and electricity, and is generally shiny

Section 9. 2 Classifying Chemical Reactions (cont. ) synthesis reaction combustion reaction decomposition reaction double-replacement reaction precipitate single-replacement reaction There are four types of chemical reactions: synthesis, combustion, decomposition, and replacement reactions.

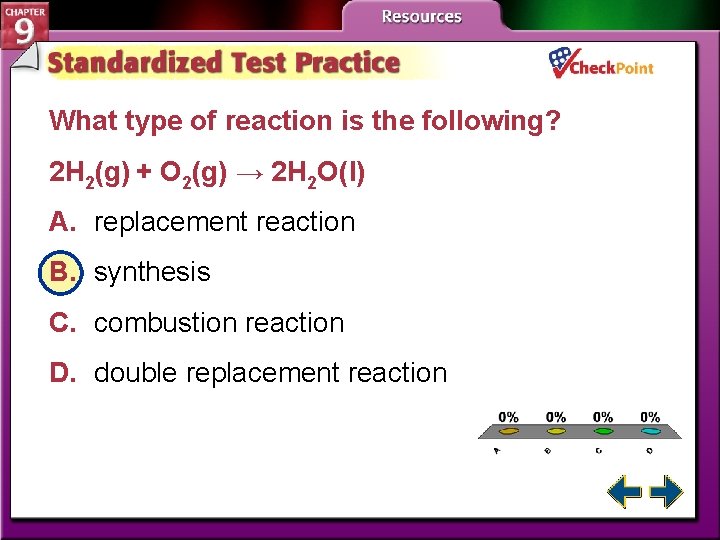
Types of Chemical Reactions • Chemists classify reactions in order to organize the many types. • A synthesis reaction is a reaction in which two or more substances react to produce a single product.

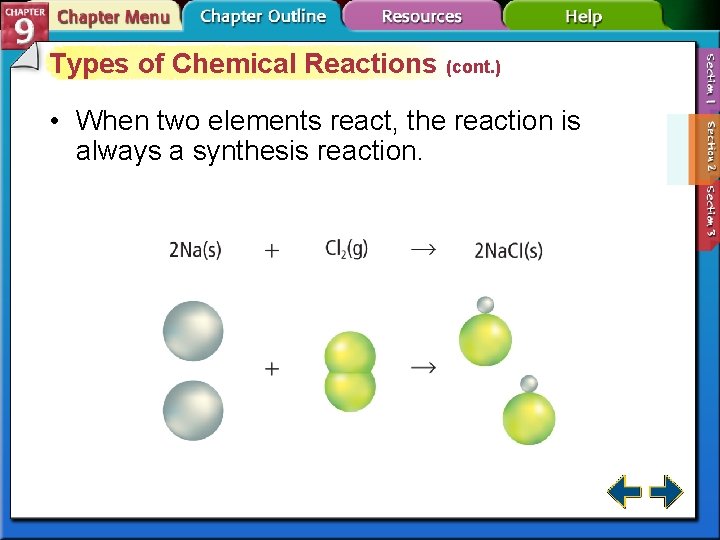
Types of Chemical Reactions (cont. ) • When two elements react, the reaction is always a synthesis reaction.

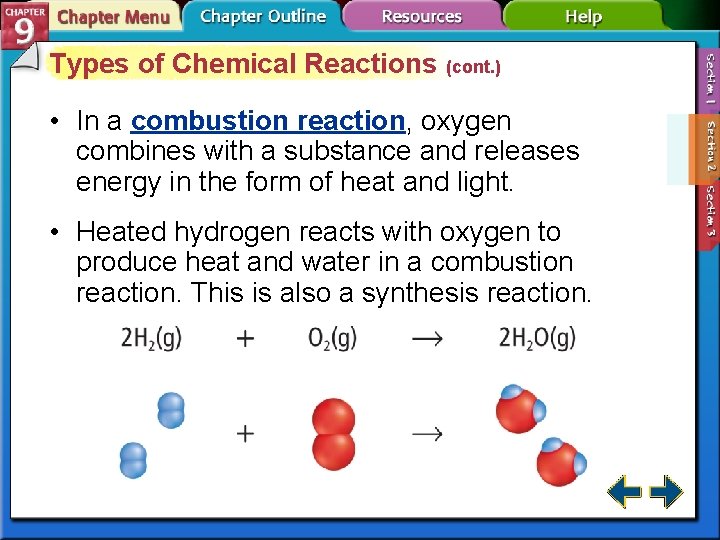
Types of Chemical Reactions (cont. ) • In a combustion reaction, oxygen combines with a substance and releases energy in the form of heat and light. • Heated hydrogen reacts with oxygen to produce heat and water in a combustion reaction. This is also a synthesis reaction.

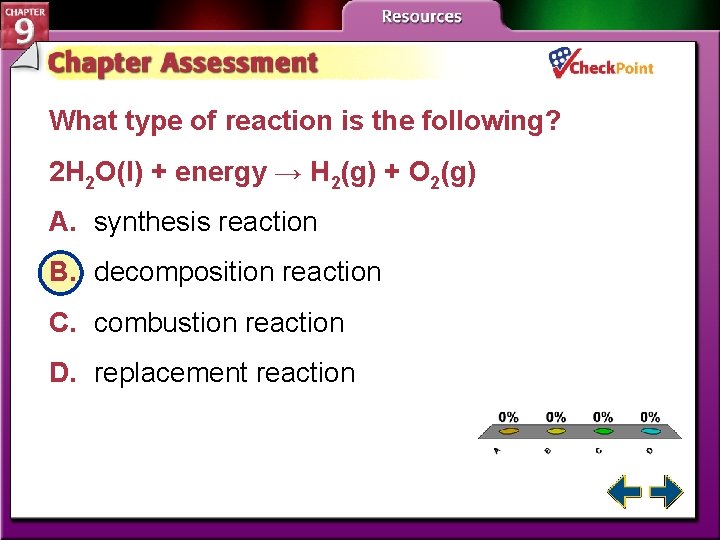
Decomposition Reactions • A decomposition reaction is one in which a single compound breaks down into two or more elements or new compounds. • Decomposition reactions often require an energy source, such as heat, light, or electricity, to occur.

Replacement Reactions • A reaction in which the atoms of one element replace the atoms of another element in a compound is called a single replacement reaction. A + BX → AX + B

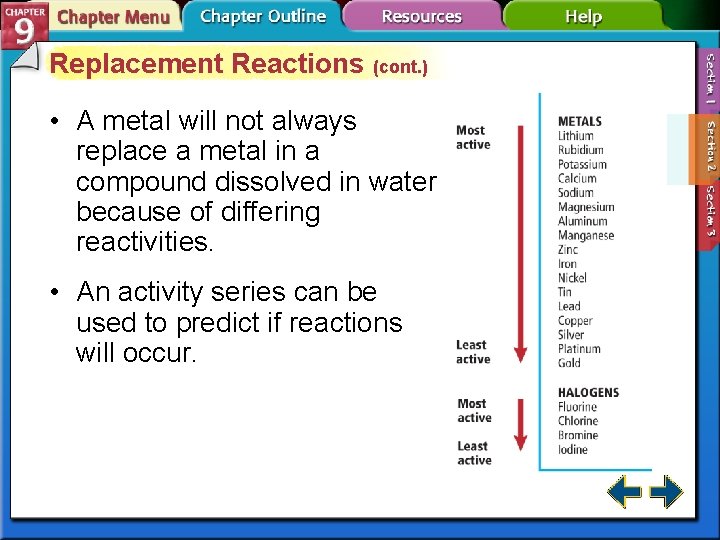
Replacement Reactions (cont. ) • A metal will not always replace a metal in a compound dissolved in water because of differing reactivities. • An activity series can be used to predict if reactions will occur.

Replacement Reactions (cont. ) • Halogens frequently replace other halogens in replacement reactions. • Halogens also have different reactivities and do not always replace each other.

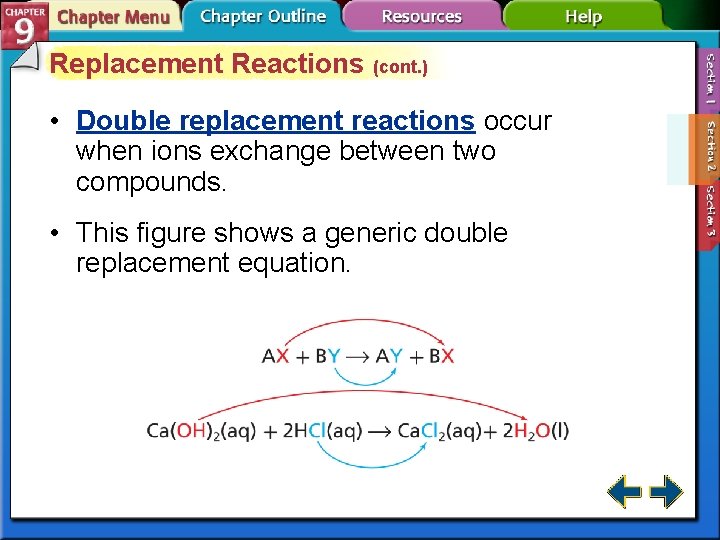
Replacement Reactions (cont. ) • Double replacement reactions occur when ions exchange between two compounds. • This figure shows a generic double replacement equation.

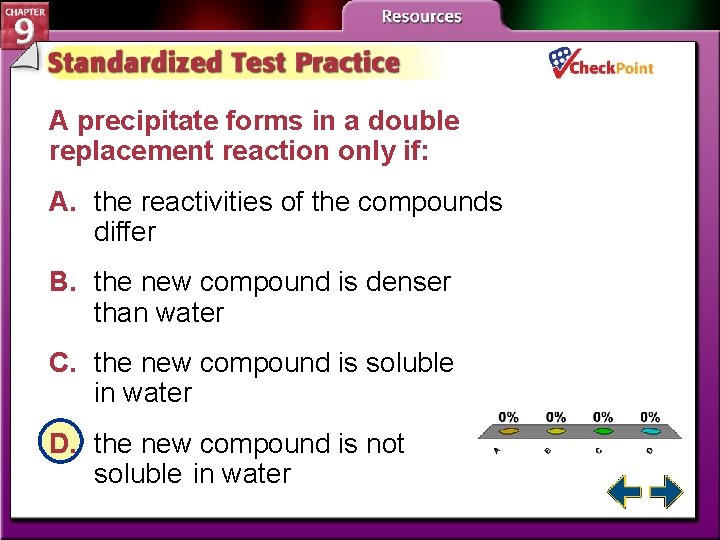
Replacement Reactions (cont. ) • The solid product produced during a chemical reaction in a solution is called a precipitate. • All double replacement reactions produce either water, a precipitate, or a gas.

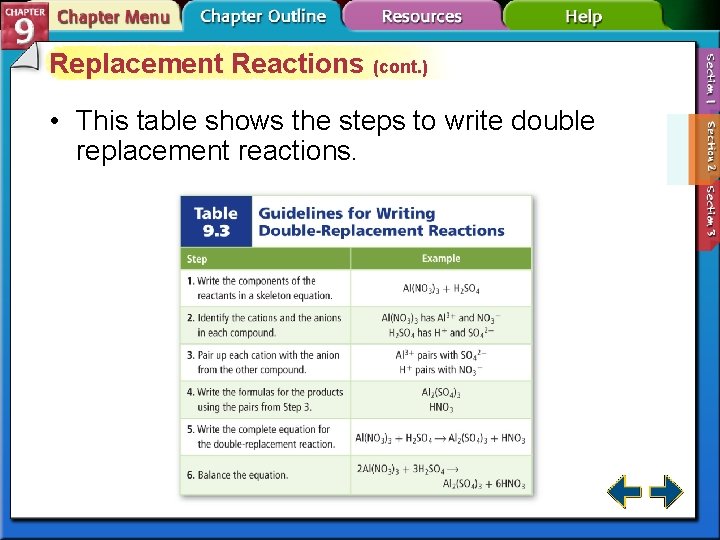
Replacement Reactions (cont. ) • This table shows the steps to write double replacement reactions.

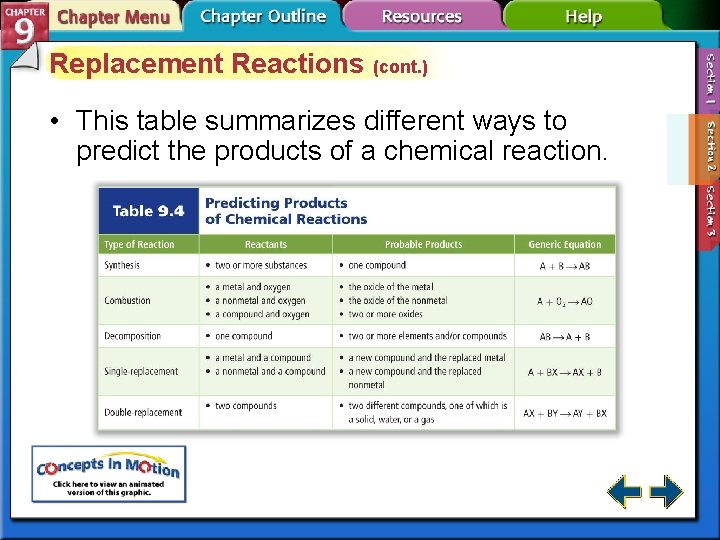
Replacement Reactions (cont. ) • This table summarizes different ways to predict the products of a chemical reaction.

Section 9. 2 Assessment Which of the following is NOT one of the four types of reactions? A. deconstructive B. synthesis C. single replacement D. double replacement A. B. C. D. A B C D

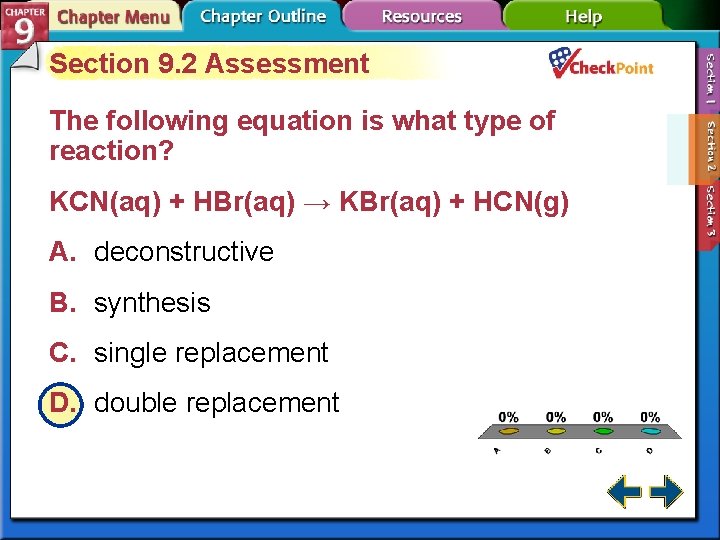
Section 9. 2 Assessment The following equation is what type of reaction? KCN(aq) + HBr(aq) → KBr(aq) + HCN(g) A. deconstructive B. synthesis C. single replacement D. double replacement A. B. C. D. A B C D


Section 9. 3 Reactions in Aqueous Solutions • Describe aqueous solutions. • Write complete ionic and net ionic equations for chemical reactions in aqueous solutions. • Predict whether reactions in aqueous solutions will produce a precipitate, water, or a gas. solution: a uniform mixture that might contain solids, liquids, or gases

Section 9. 3 Reactions in Aqueous Solutions (cont. ) aqueous solution complete ionic equation solute spectator ion solvent net ionic equation Double-replacement reactions occur between substances in aqueous solutions and produce precipitates, water, or gases.

Aqueous Solutions • An aqueous solution contains one or more dissolved substances (called solutes) in water. • The solvent is the most plentiful substance in a solution.

Aqueous Solutions (cont. ) • Water is always the solvent in an aqueous solution. • There are many possible solutes—sugar and alcohol are molecular compounds that exist as molecules in aqueous solutions. • Compounds that produce hydrogen ions in aqueous solutions are acids.

Aqueous Solutions (cont. ) • Ionic compounds can also be solutes in aqueous solutions. • When ionic compounds dissolve in water, their ions separate in a process called dissociation.

Types of Reactions in Aqueous Solutions • When two solutions that contain ions as solutes are combined, the ions might react. • If they react, it is always a double replacement reaction. • Three products can form: precipitates, water, or gases.

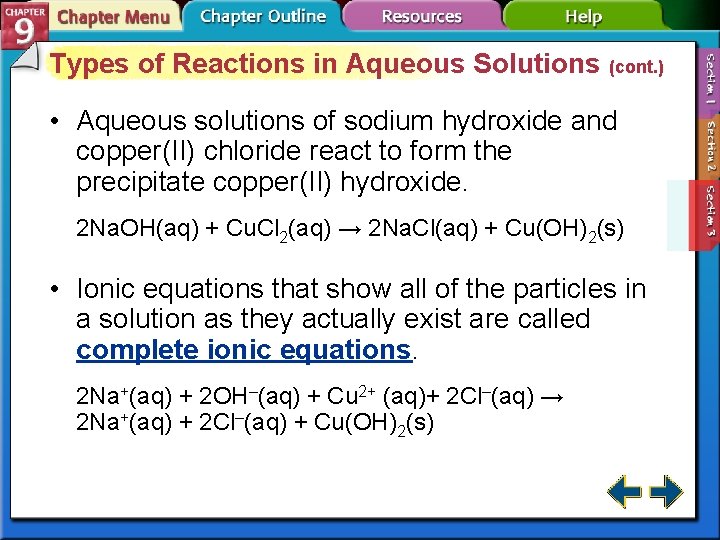
Types of Reactions in Aqueous Solutions (cont. ) • Aqueous solutions of sodium hydroxide and copper(II) chloride react to form the precipitate copper(II) hydroxide. 2 Na. OH(aq) + Cu. Cl 2(aq) → 2 Na. Cl(aq) + Cu(OH)2(s) • Ionic equations that show all of the particles in a solution as they actually exist are called complete ionic equations. 2 Na+(aq) + 2 OH–(aq) + Cu 2+ (aq)+ 2 Cl–(aq) → 2 Na+(aq) + 2 Cl–(aq) + Cu(OH)2(s)

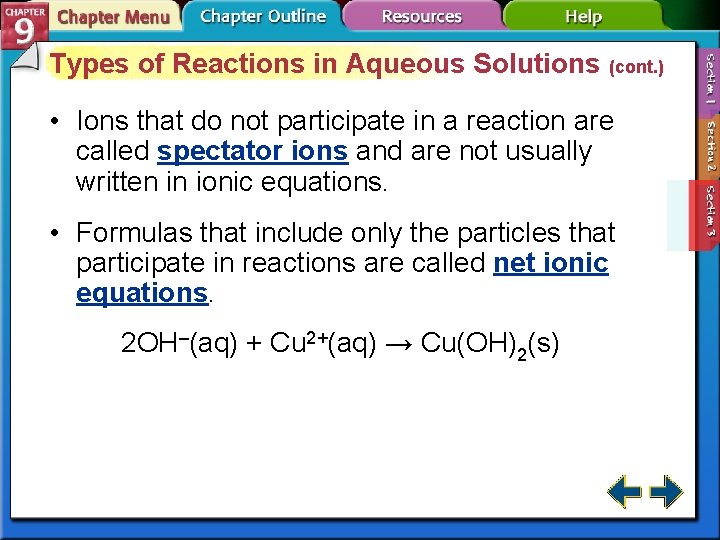
Types of Reactions in Aqueous Solutions (cont. ) • Ions that do not participate in a reaction are called spectator ions and are not usually written in ionic equations. • Formulas that include only the particles that participate in reactions are called net ionic equations. 2 OH–(aq) + Cu 2+(aq) → Cu(OH)2(s)

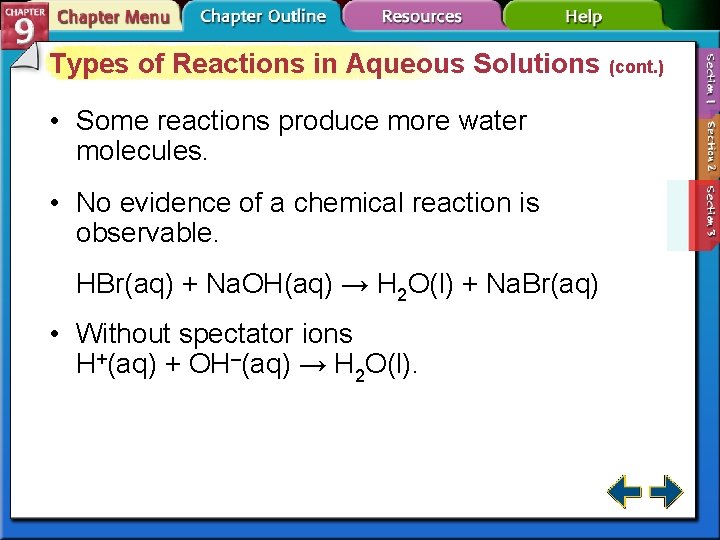
Types of Reactions in Aqueous Solutions (cont. ) • Some reactions produce more water molecules. • No evidence of a chemical reaction is observable. HBr(aq) + Na. OH(aq) → H 2 O(l) + Na. Br(aq) • Without spectator ions H+(aq) + OH–(aq) → H 2 O(l).

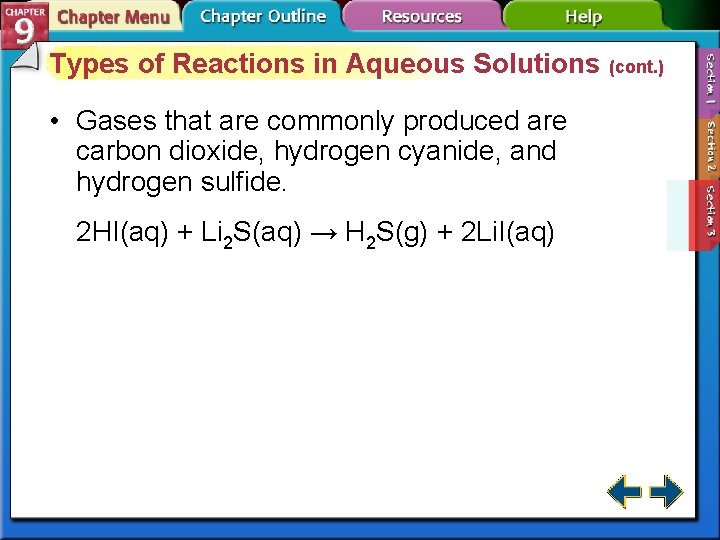
Types of Reactions in Aqueous Solutions (cont. ) • Gases that are commonly produced are carbon dioxide, hydrogen cyanide, and hydrogen sulfide. 2 HI(aq) + Li 2 S(aq) → H 2 S(g) + 2 Li. I(aq)

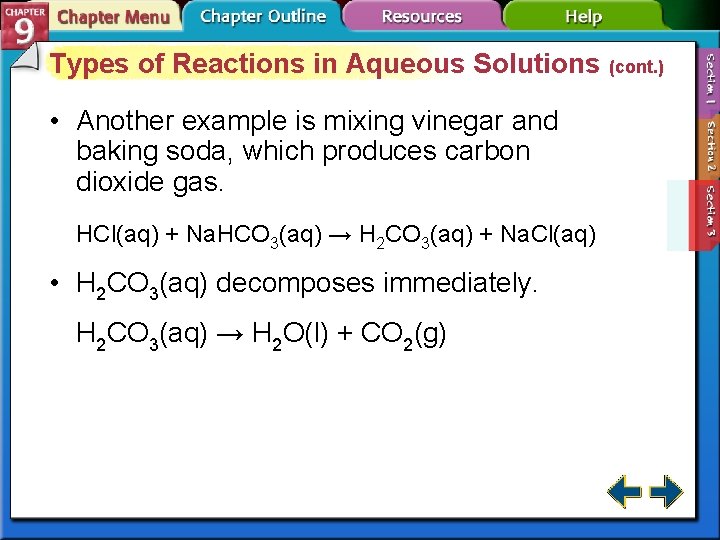
Types of Reactions in Aqueous Solutions (cont. ) • Another example is mixing vinegar and baking soda, which produces carbon dioxide gas. HCl(aq) + Na. HCO 3(aq) → H 2 CO 3(aq) + Na. Cl(aq) • H 2 CO 3(aq) decomposes immediately. H 2 CO 3(aq) → H 2 O(l) + CO 2(g)

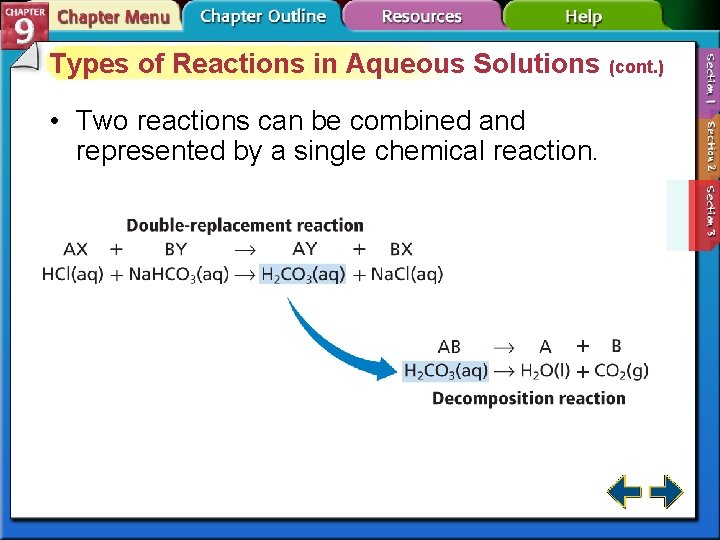
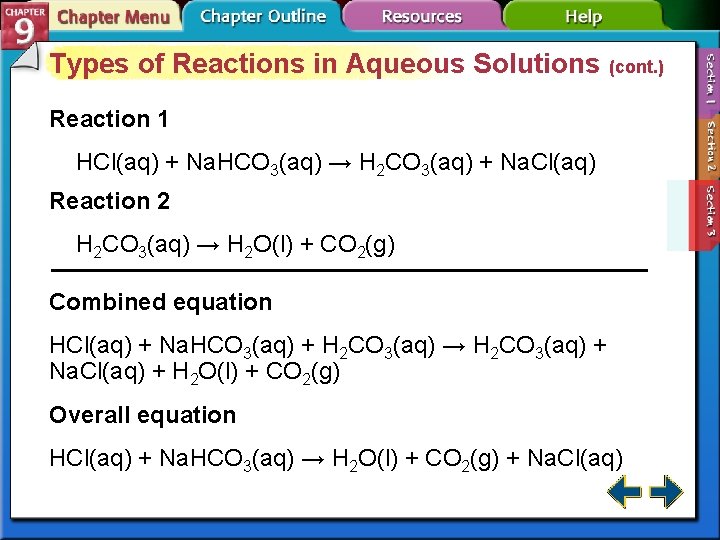
Types of Reactions in Aqueous Solutions (cont. ) • Two reactions can be combined and represented by a single chemical reaction.

Types of Reactions in Aqueous Solutions (cont. ) Reaction 1 HCl(aq) + Na. HCO 3(aq) → H 2 CO 3(aq) + Na. Cl(aq) Reaction 2 H 2 CO 3(aq) → H 2 O(l) + CO 2(g) Combined equation HCl(aq) + Na. HCO 3(aq) + H 2 CO 3(aq) → H 2 CO 3(aq) + Na. Cl(aq) + H 2 O(l) + CO 2(g) Overall equation HCl(aq) + Na. HCO 3(aq) → H 2 O(l) + CO 2(g) + Na. Cl(aq)

Section 9. 3 Assessment What is the solvent in an aqueous solution? A. hydrogen B. sodium ions C. water D. alcohol A. B. C. D. A B C D

Section 9. 3 Assessment An equation that includes only the particles that participate in a reaction is called: A. net ionic equation B. spectator ions C. complete ionic equation D. reduced ionic equation A. B. C. D. A B C D


Chemistry Online Study Guide Chapter Assessment Standardized Test Practice Image Bank Concepts in Motion

Section 9. 1 Reactions and Equations Key Concepts • Some physical changes are evidence that indicate a chemical reaction has occurred. • Word equations and skeleton equations provide important information about a chemical reaction. • A chemical equation gives the identities and relative amounts of the reactants and products that are involved in a chemical reaction. • Balancing an equation involves adjusting the coefficients until the number of atoms of each element is equal on both sides of the equation.

Section 9. 2 Classifying Chemical Reactions Key Concepts • Classifying chemical reactions makes them easier to understand, remember, and recognize. • Activity series of metals and halogens can be used to predict if single-replacement reactions will occur.

Section 9. 3 Reactions in Aqueous Solutions Key Concepts • In aqueous solutions, the solvent is always water. There are many possible solutes. • Many molecular compounds form ions when they dissolve in water. When some ionic compounds dissolve in water, their ions separate. • When two aqueous solutions that contain ions as solutes are combined, the ions might react with one another. The solvent molecules do not usually react. • Reactions that occur in aqueous solutions are doublereplacement reactions.

The law of conservation of mass requires what in a chemical reaction equation? A. both sides of the equation to contain the same substances B. the reactants to have the same amount of molecules as the products C. both sides to have the same amount of atoms of each element D. the products to have fewer molecules than the reactants A. B. C. D. A B C D

A reaction that gives off heat is what type of reaction? A. single replacement reaction B. double replacement reaction C. synthesis reaction D. combustion reaction A. B. C. D. A B C D

Ions that are present in a solution and do not participate in a chemical reaction when another substance is added are called ____. A. spectator ions B. reactants C. products D. net ions A. B. C. D. A B C D

A double replacement reaction produces all of the following except ____. A. gases B. solids C. light D. water A. B. C. D. A B C D

What type of reaction is the following? 2 H 2 O(l) + energy → H 2(g) + O 2(g) A. synthesis reaction B. decomposition reaction C. combustion reaction D. replacement reaction A. B. C. D. A B C D

What type of reaction is the following? 2 H 2(g) + O 2(g) → 2 H 2 O(l) A. replacement reaction B. synthesis C. combustion reaction D. double replacement reaction A. B. C. D. A B C D

A precipitate forms in a double replacement reaction only if: A. the reactivities of the compounds differ B. the new compound is denser than water C. the new compound is soluble in water D. the new compound is not soluble in water A. B. C. D. A B C D

A ____ is a statement that uses chemical formulas to show the identities and relative amounts of the substances involved in a chemical reaction. A. word equation B. skeleton equation C. chemical equation D. balanced equation A. B. C. D. A B C D

Predict the type of reaction. Li. Br 2 (aq) + 2 Na. OH (aq) → ____ A. synthesis reaction B. combustion reaction C. single replacement reaction D. double replacement reaction A. B. C. D. A B C D

Which reactions are essentially the opposite of synthesis reactions? A. single-replacement B. decomposition C. combustion D. double-replacement A. B. C. D. A B C D

Click on an image to enlarge.













Table 9. 2 Steps for Balancing Equations Figure 9. 15 The Forming of a Precipitate Table 9. 4 Types of Chemical Reactions

Click any of the background top tabs to display the respective folder. Within the Chapter Outline, clicking a section tab on the right side of the screen will bring you to the first slide in each respective section. Simple navigation buttons will allow you to progress to the next slide or the previous slide. The Chapter Resources Menu will allow you to access chapter specific resources from the Chapter Menu or any Chapter Outline slide. From within any feature, click the Resources tab to return to this slide. The “Return” button will allow you to return to the slide that you were viewing when you clicked either the Resources or Help tab. To exit the presentation, click the Exit button on the Chapter Menu slide or hit Escape [Esc] on your keyboards while viewing any Chapter Outline slide.

This slide is intentionally blank.
 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Types of reactions
Types of reactions Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Section 1 chemical changes
Section 1 chemical changes Chemical equations and reactions chapter 8
Chemical equations and reactions chapter 8 Balancing chemical equations definition
Balancing chemical equations definition Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Lesson 68 toxic reactions chemical equations
Lesson 68 toxic reactions chemical equations Synthesis reaction
Synthesis reaction Toxic reactions chemical equations
Toxic reactions chemical equations Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Combination reaction equation
Combination reaction equation Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Section 2 chemical reactions answer key
Section 2 chemical reactions answer key Translate word equations to chemical equations
Translate word equations to chemical equations Balancing redox reactions in acidic solution
Balancing redox reactions in acidic solution Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Chemical reactions reactants and products
Chemical reactions reactants and products Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Percent yield of copper
Percent yield of copper Building vocabulary: chemical bonds and reactions
Building vocabulary: chemical bonds and reactions Proportional relationships in chemical reactions
Proportional relationships in chemical reactions Types of redox reactions
Types of redox reactions Types of reaction
Types of reaction Types of reactions chemistry
Types of reactions chemistry Tyoes of chemical reactions
Tyoes of chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions Chapter 10 chemical reactions answer key
Chapter 10 chemical reactions answer key The calculations of quantities in chemical reactions
The calculations of quantities in chemical reactions Principles of immuno chemical reactions
Principles of immuno chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions More predicting products of chemical reactions
More predicting products of chemical reactions Unit 11 chemical reactions
Unit 11 chemical reactions Four types of chemical reactions
Four types of chemical reactions What is the role of enzymes in chemical reactions
What is the role of enzymes in chemical reactions Describing chemical reactions
Describing chemical reactions Chemical reactions classification
Chemical reactions classification Examples of chemical reactions in everyday life
Examples of chemical reactions in everyday life 5 general types of chemical reactions
5 general types of chemical reactions 5 general types of chemical reactions
5 general types of chemical reactions Chemical reactions study guide
Chemical reactions study guide Chapter 9 chemical reactions answers
Chapter 9 chemical reactions answers Equilibrium of chemical reactions
Equilibrium of chemical reactions Chapter 9 chemical reactions
Chapter 9 chemical reactions What are the 4 types of chemical reactions
What are the 4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions Chemical reactions chapter 9 study guide
Chemical reactions chapter 9 study guide 5 chemical reactions
5 chemical reactions Examples of double replacement reactions
Examples of double replacement reactions Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Chapter 11 chemical reactions practice problems
Chapter 11 chemical reactions practice problems Physical properties of esters
Physical properties of esters Chemical reactions summary
Chemical reactions summary Chemical reactions in bread
Chemical reactions in bread Solvent in chemical reactions
Solvent in chemical reactions Three types of chemical reactions
Three types of chemical reactions Mass relationships in chemical reactions
Mass relationships in chemical reactions Indications of a chemical reaction
Indications of a chemical reaction Describing chemical reactions
Describing chemical reactions Rules of chemical reaction
Rules of chemical reaction Chemical reaction rearrangement of atoms
Chemical reaction rearrangement of atoms Classification of chemical reactions worksheet
Classification of chemical reactions worksheet What are the five general types of chemical reactions
What are the five general types of chemical reactions Chemical reactions in welding
Chemical reactions in welding Chemistry unit 4 grade 11
Chemistry unit 4 grade 11 A balanced chemical reaction obeys the law of
A balanced chemical reaction obeys the law of Understanding chemical reactions worksheet answer key
Understanding chemical reactions worksheet answer key Stoichiometry map for chemical reactions
Stoichiometry map for chemical reactions Which macromolecule speeds up chemical reactions
Which macromolecule speeds up chemical reactions Inorganic non aqueous solvents
Inorganic non aqueous solvents Are all chemical reactions reversible
Are all chemical reactions reversible Chemical reactions in water
Chemical reactions in water Solvent in chemical reactions
Solvent in chemical reactions