Chemical Reactions Quantitative Chem Indicators of a Chemical





- Slides: 28

Chemical Reactions Quantitative Chem

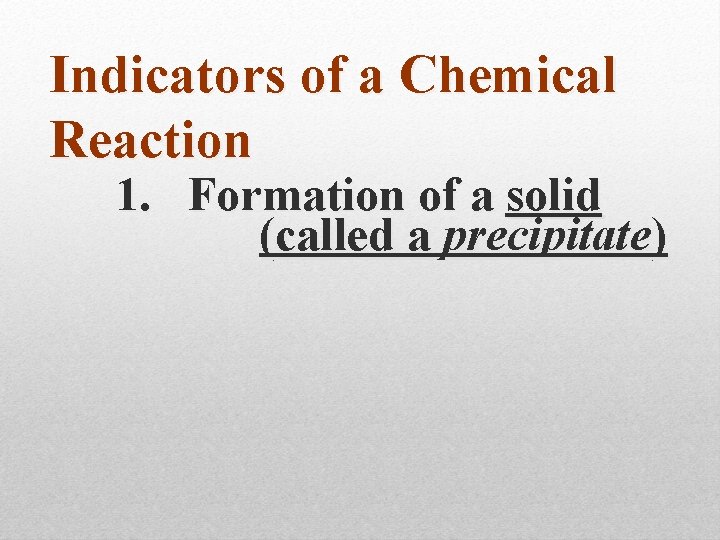
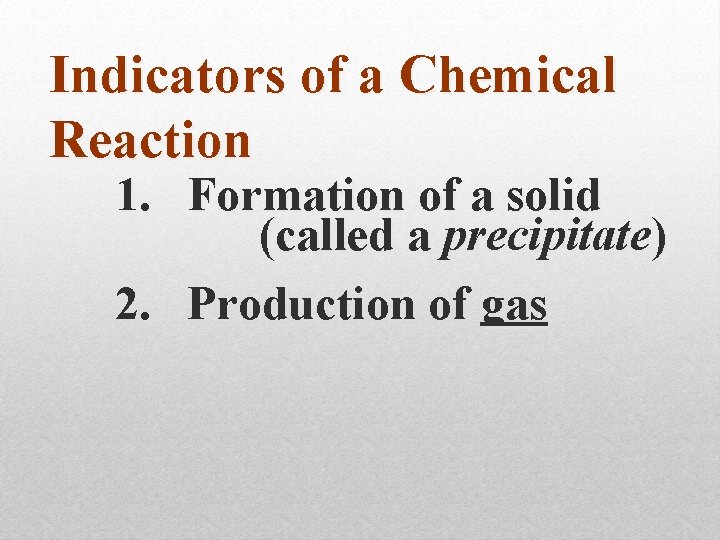
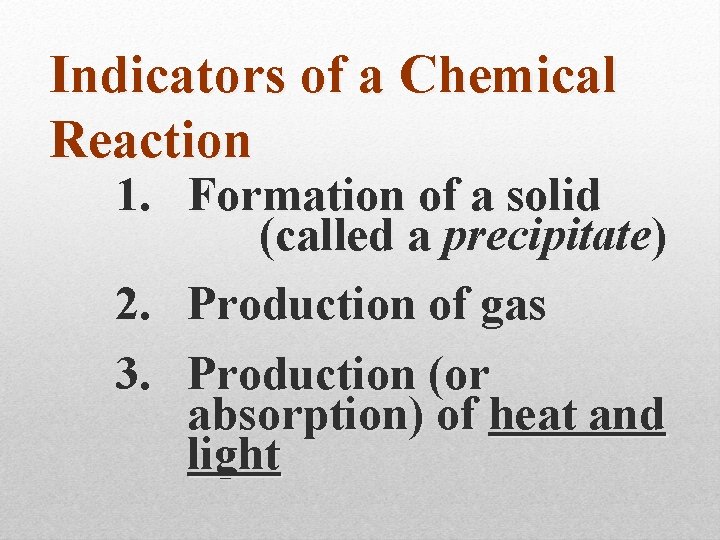
Indicators of a Chemical Reaction

Indicators of a Chemical Reaction 1. Formation of a solid (called a precipitate)

Indicators of a Chemical Reaction 1. Formation of a solid (called a precipitate) 2. Production of gas

Indicators of a Chemical Reaction 1. Formation of a solid (called a precipitate) 2. Production of gas 3. Production (or absorption) of heat and light

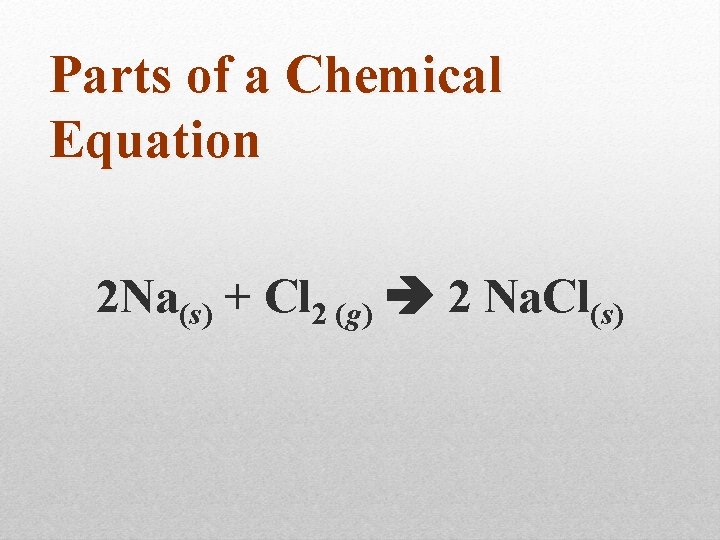
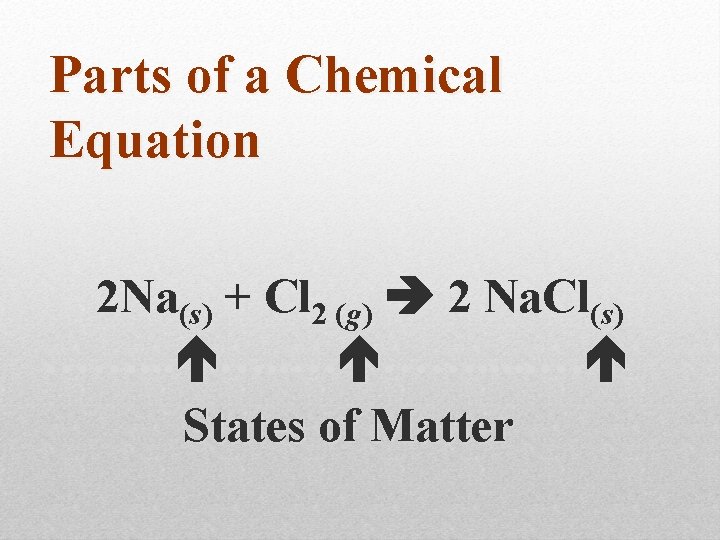
Parts of a Chemical Equation 2 Na(s) + Cl 2 (g) 2 Na. Cl(s)

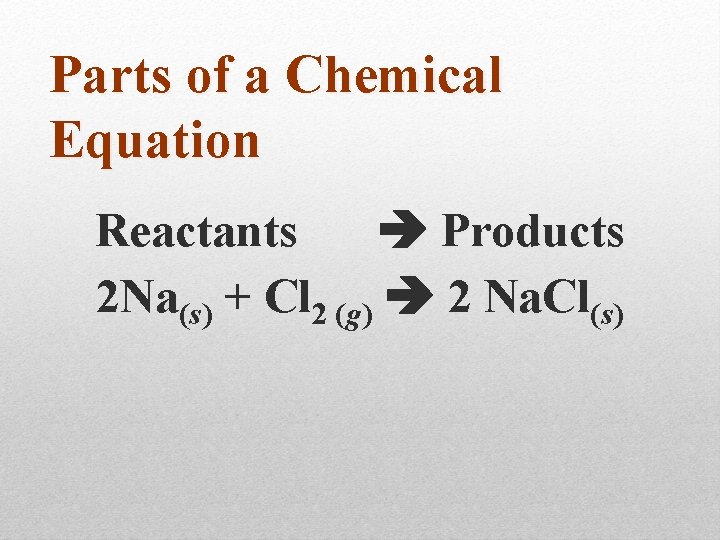
Parts of a Chemical Equation Reactants Products 2 Na(s) + Cl 2 (g) 2 Na. Cl(s)

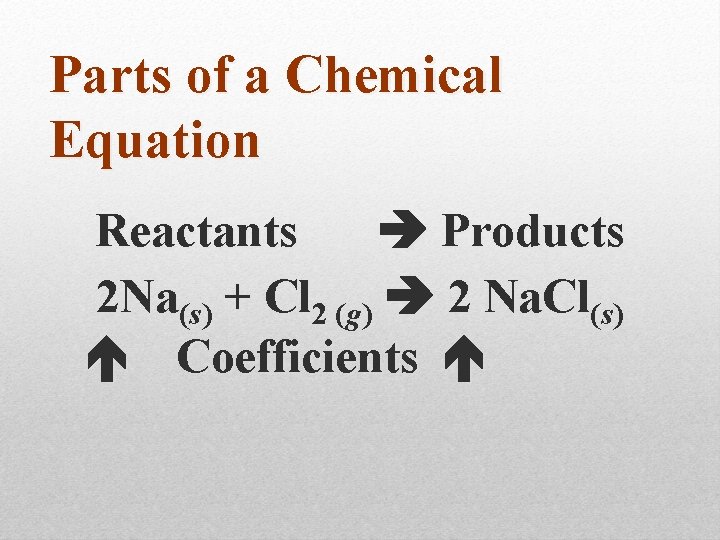
Parts of a Chemical Equation Reactants Products 2 Na(s) + Cl 2 (g) 2 Na. Cl(s) Coefficients

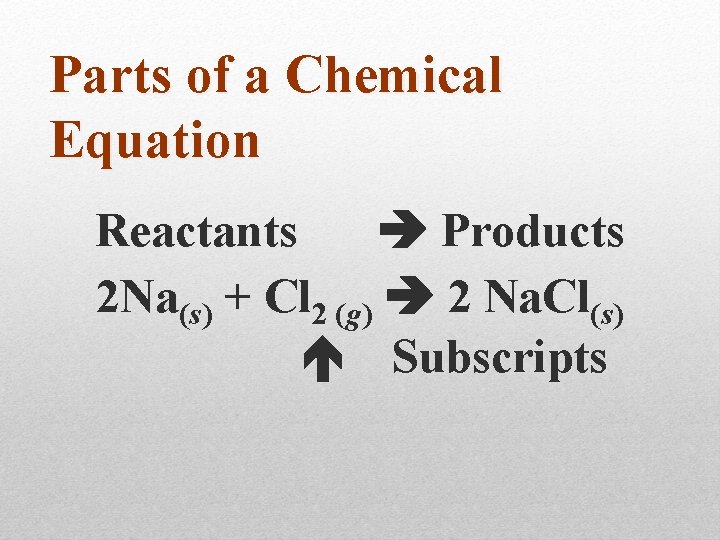
Parts of a Chemical Equation Reactants Products 2 Na(s) + Cl 2 (g) 2 Na. Cl(s) Subscripts

Parts of a Chemical Equation 2 Na(s) + Cl 2 (g) 2 Na. Cl(s) ……. . . … States of Matter

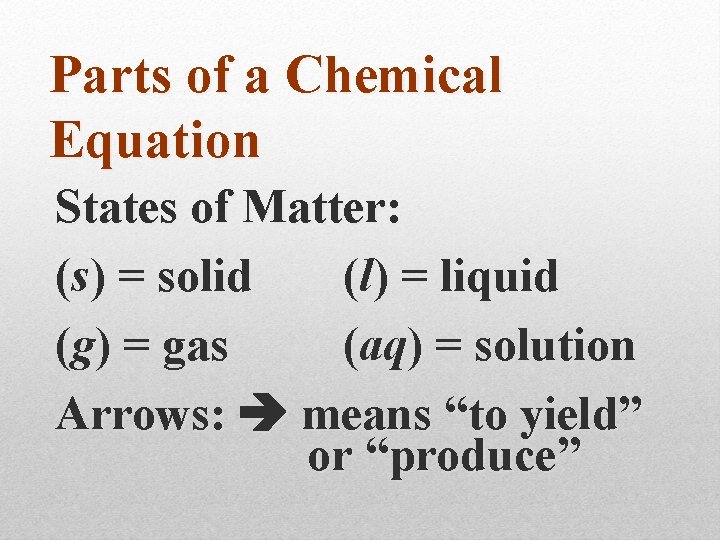
Parts of a Chemical Equation States of Matter: (s) = solid (l) = liquid (g) = gas (aq) = solution Arrows: means “to yield” or “produce”

Why do we need to balance a chemical equation? Because matter is not created or destroyed in a chemical reaction! (The law of conservation of matter) The amount of each element remains the same.

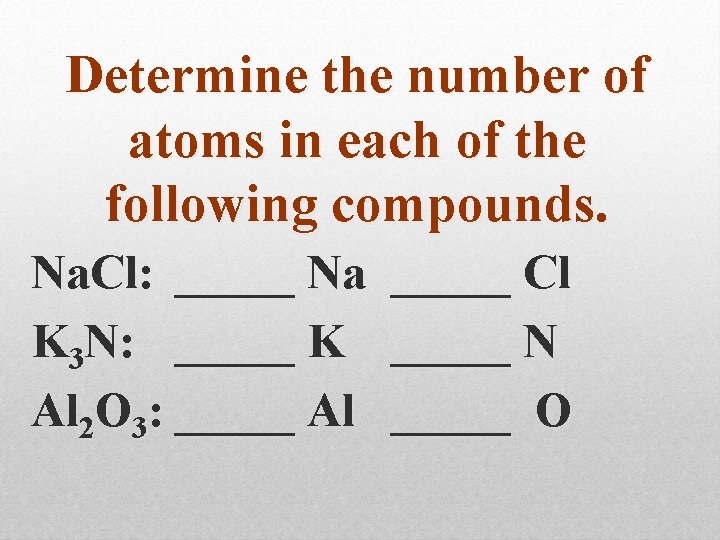
Determine the number of atoms in each of the following compounds. Na. Cl: _____ Na K 3 N: _____ K Al 2 O 3: _____ Al _____ Cl _____ N _____ O

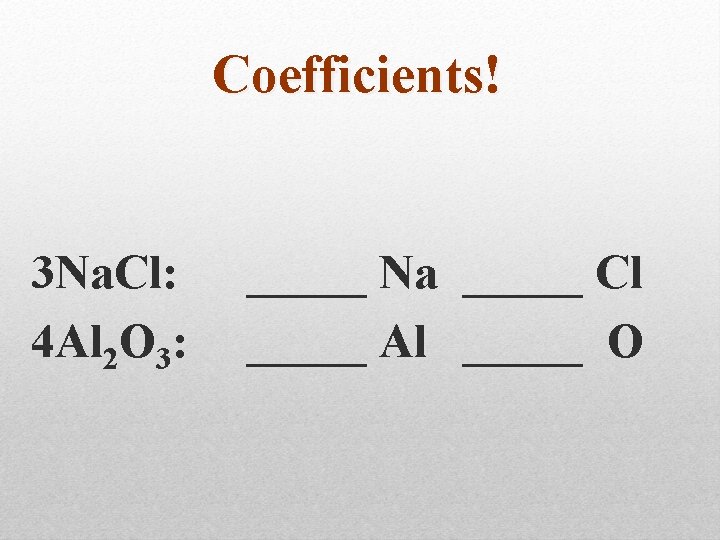
Coefficients! 3 Na. Cl: 4 Al 2 O 3: _____ Na _____ Cl _____ Al _____ O

Rules for Balancing (Coeff) 1. Start with the most complex element or compound. 2. Balance oxygen last 3. Balance hydrogen second to last 4. If stuck on oxygen, double everything except O 2, then balance. 5. Double-check your work.

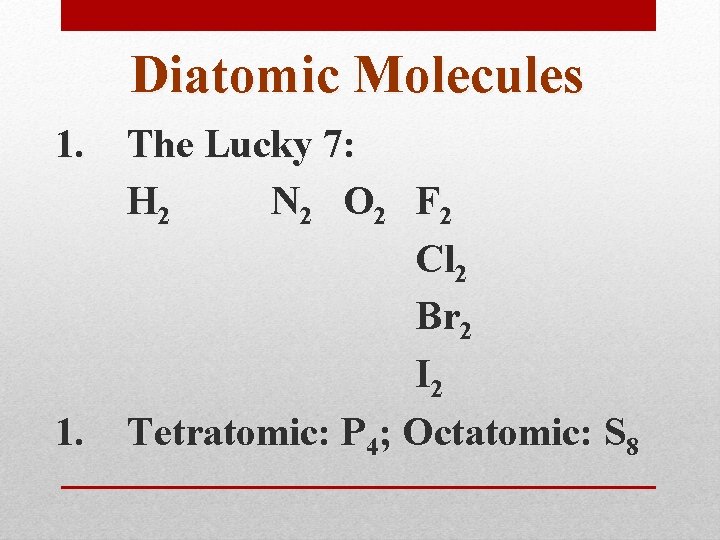
Diatomic Molecules 1. The Lucky 7: H 2 N 2 O 2 F 2 Cl 2 Br 2 I 2 1. Tetratomic: P 4; Octatomic: S 8

Steps to write chemical eqns 1. Write the elements correctly. 2. Write the compounds correctly by balancing their charges (Exception: Type III cmpds) 3. Balance the chemical equation.

3. Classifying Reactions and Predicting Products

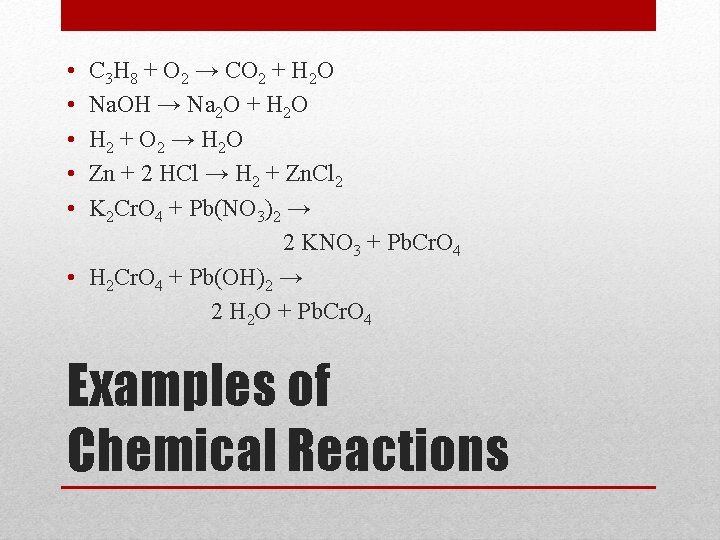
• • • C 3 H 8 + O 2 → CO 2 + H 2 O Na. OH → Na 2 O + H 2 O H 2 + O 2 → H 2 O Zn + 2 HCl → H 2 + Zn. Cl 2 K 2 Cr. O 4 + Pb(NO 3)2 → 2 KNO 3 + Pb. Cr. O 4 • H 2 Cr. O 4 + Pb(OH)2 → 2 H 2 O + Pb. Cr. O 4 Examples of Chemical Reactions

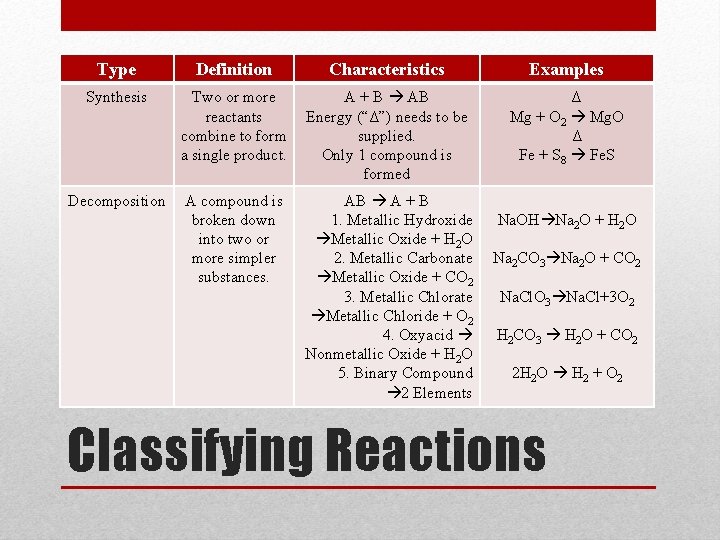
Type Definition Characteristics Examples Synthesis Two or more reactants combine to form a single product. A + B AB Energy (“Δ”) needs to be supplied. Only 1 compound is formed Δ Mg + O 2 Mg. O Δ Fe + S 8 Fe. S Decomposition A compound is broken down into two or more simpler substances. AB A + B 1. Metallic Hydroxide Metallic Oxide + H 2 O 2. Metallic Carbonate Metallic Oxide + CO 2 3. Metallic Chlorate Metallic Chloride + O 2 4. Oxyacid Nonmetallic Oxide + H 2 O 5. Binary Compound 2 Elements Na. OH Na 2 O + H 2 O Na 2 CO 3 Na 2 O + CO 2 Na. Cl. O 3 Na. Cl+3 O 2 H 2 CO 3 H 2 O + CO 2 2 H 2 O H 2 + O 2 Classifying Reactions

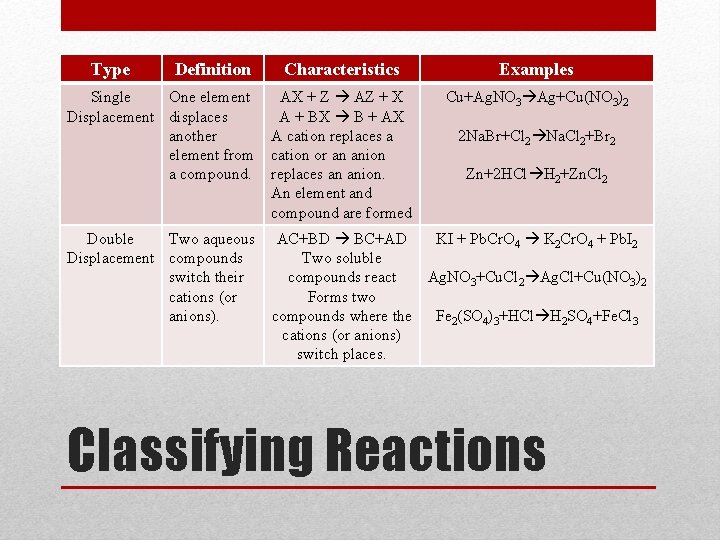
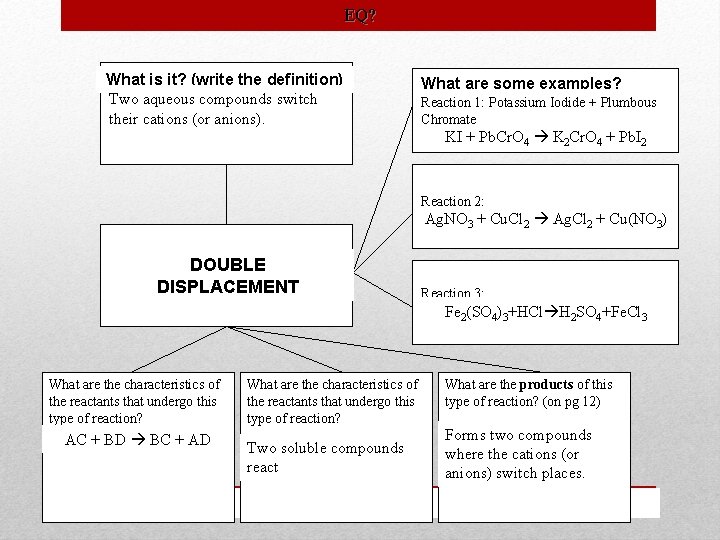
Type Definition Characteristics Examples Single One element Displacement displaces another element from a compound. AX + Z AZ + X A + BX B + AX A cation replaces a cation or an anion replaces an anion. An element and compound are formed Cu+Ag. NO 3 Ag+Cu(NO 3)2 Double Two aqueous Displacement compounds switch their cations (or anions). AC+BD BC+AD KI + Pb. Cr. O 4 K 2 Cr. O 4 + Pb. I 2 Two soluble compounds react Ag. NO 3+Cu. Cl 2 Ag. Cl+Cu(NO 3)2 Forms two compounds where the Fe 2(SO 4)3+HCl H 2 SO 4+Fe. Cl 3 cations (or anions) switch places. 2 Na. Br+Cl 2 Na. Cl 2+Br 2 Zn+2 HCl H 2+Zn. Cl 2 Classifying Reactions

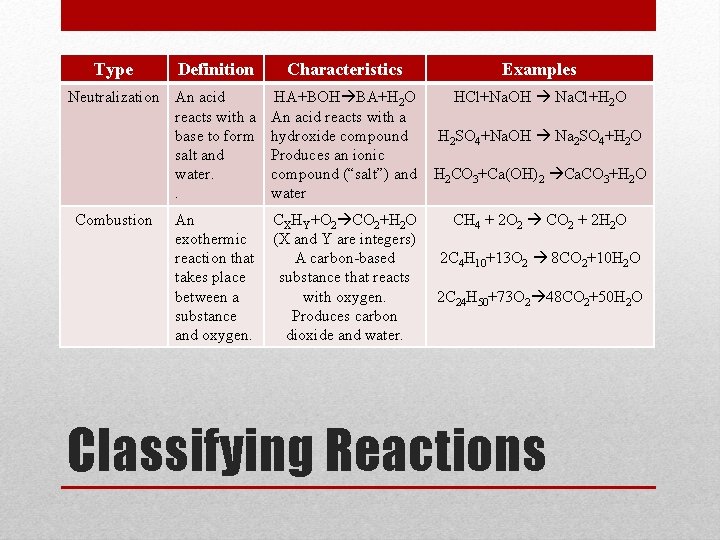
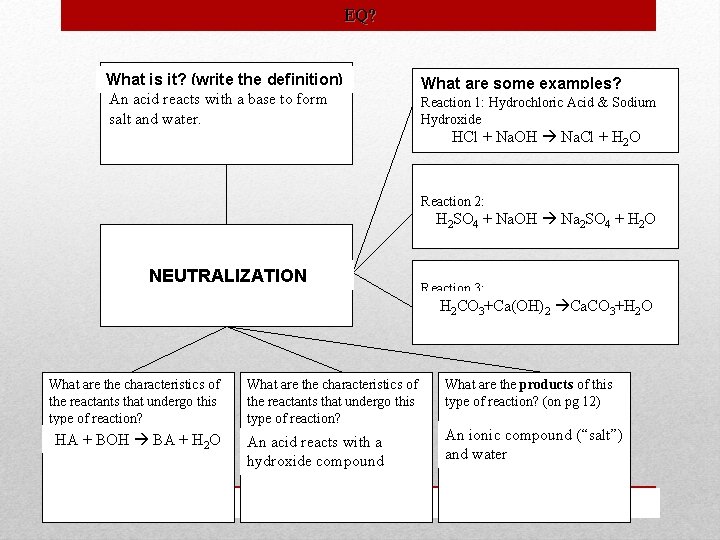
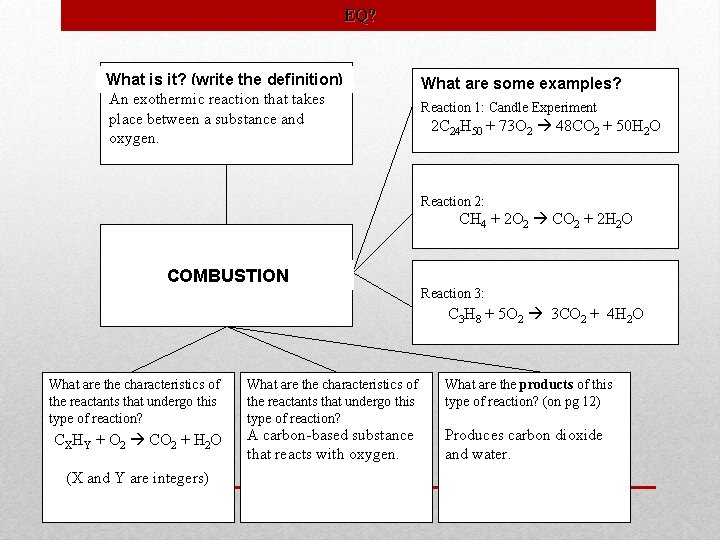
Type Definition Neutralization An acid reacts with a base to form salt and water. . Combustion An exothermic reaction that takes place between a substance and oxygen. Characteristics Examples HA+BOH BA+H 2 O An acid reacts with a hydroxide compound Produces an ionic compound (“salt”) and water HCl+Na. OH Na. Cl+H 2 O CXHY+O 2 CO 2+H 2 O (X and Y are integers) A carbon-based substance that reacts with oxygen. Produces carbon dioxide and water. H 2 SO 4+Na. OH Na 2 SO 4+H 2 O H 2 CO 3+Ca(OH)2 Ca. CO 3+H 2 O CH 4 + 2 O 2 CO 2 + 2 H 2 O 2 C 4 H 10+13 O 2 8 CO 2+10 H 2 O 2 C 24 H 50+73 O 2 48 CO 2+50 H 2 O Classifying Reactions

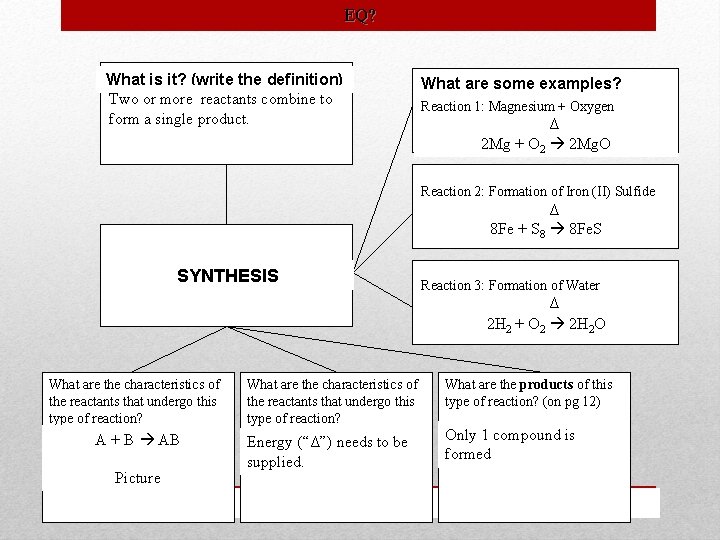
EQ? What is it? (write the definition) Two or more reactants combine to form a single product. What are some examples? Reaction 1: Magnesium + Oxygen Δ 2 Mg + O 2 2 Mg. O Reaction 2: Formation of Iron (II) Sulfide Δ 8 Fe + S 8 8 Fe. S SYNTHESIS Reaction 3: Formation of Water Δ 2 H 2 H HH 22++OO H 2 O 2 2 2. O What are the characteristics of the reactants that undergo this type of reaction? A + B AB Picture What are the characteristics of the reactants that undergo this type of reaction? Energy (“Δ”) needs to be supplied. What are the products of this type of reaction? (on pg 12) Only 1 compound is formed

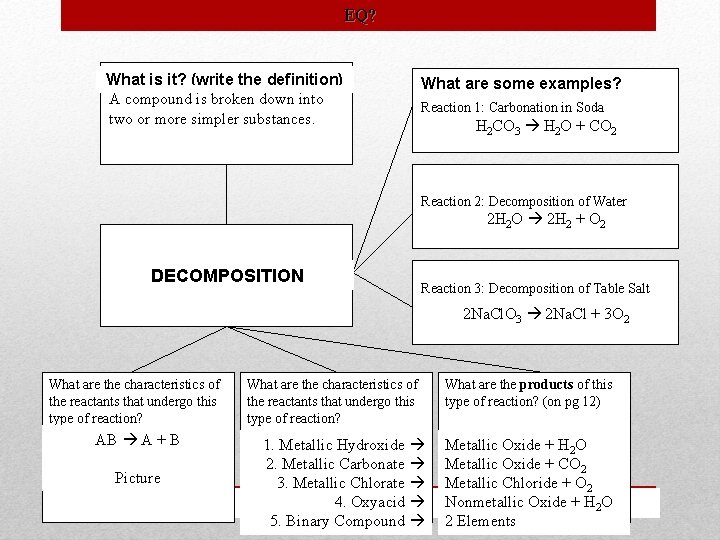
EQ? What is it? (write the definition) A compound is broken down into two or more simpler substances. What are some examples? Reaction 1: Carbonation in Soda H 2 CO 3 H 2 O + CO 2 Reaction 2: Decomposition of Water 2 H 2 O 2 H 2 + O 2 DECOMPOSITION Reaction 3: Decomposition of Table Salt Na. Cl. O 3 Na. Cl ++O 3 O. Na. Cl. O 2 2 33 2 Na. Cl What are the characteristics of the reactants that undergo this type of reaction? AB A + B Picture What are the characteristics of the reactants that undergo this type of reaction? 1. Metallic Hydroxide 2. Metallic Carbonate 3. Metallic Chlorate 4. Oxyacid 5. Binary Compound What are the products of this type of reaction? (on pg 12) Metallic Oxide + H 2 O Metallic Oxide + CO 2 Metallic Chloride + O 2 Nonmetallic Oxide + H 2 O 2 Elements

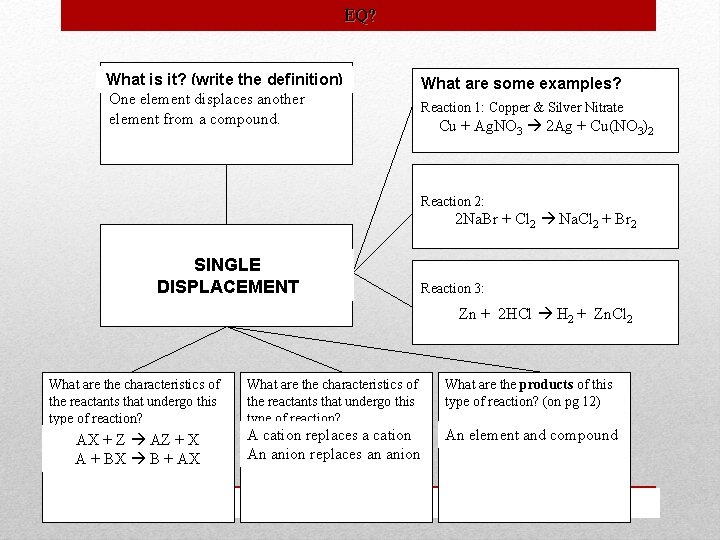
EQ? What is it? (write the definition) One element displaces another element from a compound. What are some examples? Reaction 1: Copper & Silver Nitrate Cu + Ag. NO 3 2 Ag + Cu(NO 3)2 Reaction 2: 2 Na. Br + Cl 2 Na. Cl 2 + Br 2 SINGLE DISPLACEMENT Reaction 3: Zn HCl Zn++ + 2 HCl HH 222+ ++ Zn. Cl Zn H Zn. Cl 222 What are the characteristics of the reactants that undergo this type of reaction? What are the products of this type of reaction? (on pg 12) AX + Z AZ + X A + BX B + AX A cation replaces a cation An anion replaces an anion An element and compound

EQ? What is it? (write the definition) Two aqueous compounds switch their cations (or anions). What are some examples? Reaction 1: Potassium Iodide + Plumbous Chromate KI + Pb. Cr. O 4 K 2 Cr. O 4 + Pb. I 2 Reaction 2: Ag. NO 3 + Cu. Cl 2 Ag. Cl 2 + Cu(NO 3) DOUBLE DISPLACEMENT Reaction 3: Fe 2(SO Fe 42)(SO 3+HCl H 2 SO 4+Fe. Cl 3 4)3 + HCl What are the characteristics of the reactants that undergo this type of reaction? AC + BD BC + AD What are the characteristics of the reactants that undergo this type of reaction? Two soluble compounds react What are the products of this type of reaction? (on pg 12) Forms two compounds where the cations (or anions) switch places.

EQ? What is it? (write the definition) An acid reacts with a base to form salt and water. What are some examples? Reaction 1: Hydrochloric Acid & Sodium Hydroxide HCl + Na. OH Na. Cl + H 2 O Reaction 2: H 2 SO 4 + Na. OH Na 2 SO 4 + H 2 O NEUTRALIZATION Reaction 3: H 2 COH 3+Ca(OH) 2 Ca. CO 3+H 2 O 2 CO 3 + Ca(OH) 2 What are the characteristics of the reactants that undergo this type of reaction? HA + BOH BA + H 2 O What are the characteristics of the reactants that undergo this type of reaction? What are the products of this type of reaction? (on pg 12) An acid reacts with a hydroxide compound An ionic compound (“salt”) and water

EQ? What is it? (write the definition) An exothermic reaction that takes place between a substance and oxygen. What are some examples? Reaction 1: Candle Experiment 2 C 24 H 50 + 73 O 2 48 CO 2 + 50 H 2 O Reaction 2: CH 4 + 2 O 2 CO 2 + 2 H 2 O COMBUSTION Reaction 3: O 222 3 CO CO 22++ H 4 H CC 5 O 33 H 88 + 2 O 2. O 3 H 8+ What are the characteristics of the reactants that undergo this type of reaction? What are the products of this type of reaction? (on pg 12) CXHY + O 2 CO 2 + H 2 O A carbon-based substance that reacts with oxygen. Produces carbon dioxide and water. (X and Y are integers)