Chemical Reactions Proof of rxn and Activity Series
















- Slides: 22

Chemical Reactions Proof of rxn and Activity Series

1/14/13 Bell Work

Indications of a Chemical Reaction Let me give you some hints.

Is it a chemical reaction? C. Johannesson If a gas is produced it might be a chemical reaction. Look for the bubbles!

Is it a chemical reaction? C. Johannesson If a precipitate is formed it might be a chemical reaction. A solid is left behind.

Is it a chemical reaction? C. Johannesson If it changes color it might be a chemical reaction. Like iron rusting!

Is it a chemical reaction? C. Johannesson If evolution of heat and light, it might be a chemical reaction.

Is it a chemical reaction? C. Johannesson Or…if it gets colder it might be a chemical reaction.

Energy in Reactions • Endothermic. Energy moves from the surroundings into the source EX: Cold packs, Alka-seltzer • Exothermic. Energy moves from the source out to the surroundings EX: Light a match, fuel

3 Types of Reactions • Redox Reactions • Precipitate Reactions • Acid-Base Reactions

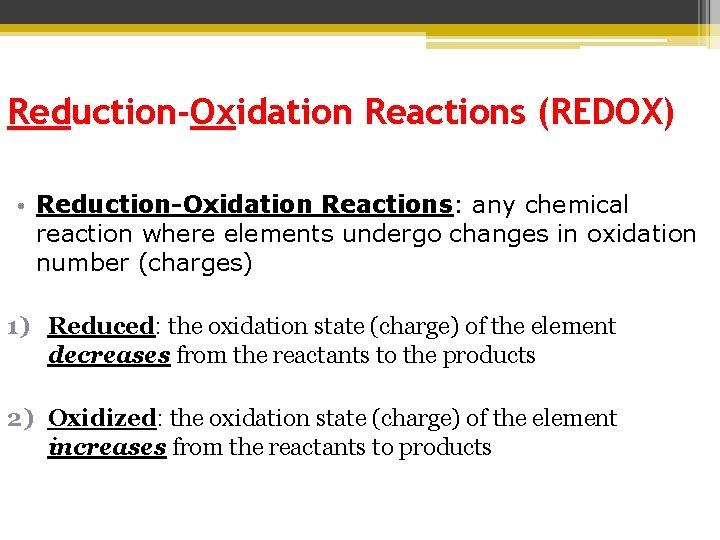
Reduction-Oxidation Reactions (REDOX) • Reduction-Oxidation Reactions: any chemical reaction where elements undergo changes in oxidation number (charges) 1) Reduced: the oxidation state (charge) of the element decreases from the reactants to the products 2) Oxidized: the oxidation state (charge) of the element increases from the reactants to products

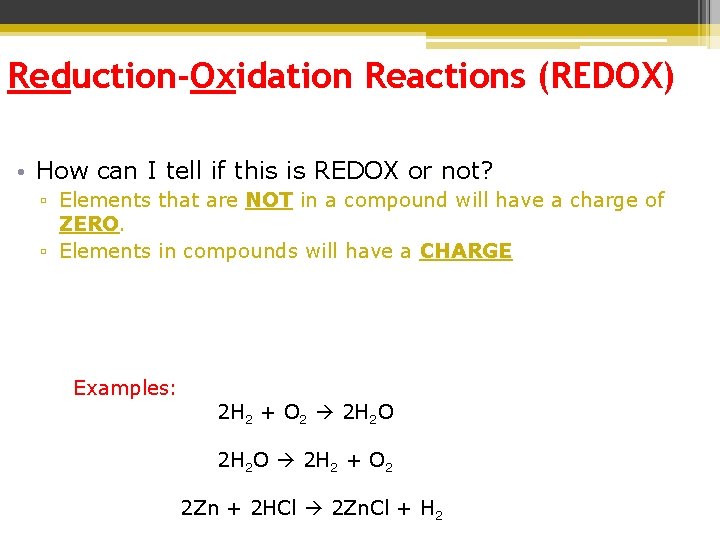
Reduction-Oxidation Reactions (REDOX) • How can I tell if this is REDOX or not? ▫ Elements that are NOT in a compound will have a charge of ZERO. ▫ Elements in compounds will have a CHARGE Examples: 2 H 2 + O 2 2 H 2 O 2 H 2 + O 2 2 Zn + 2 HCl 2 Zn. Cl + H 2

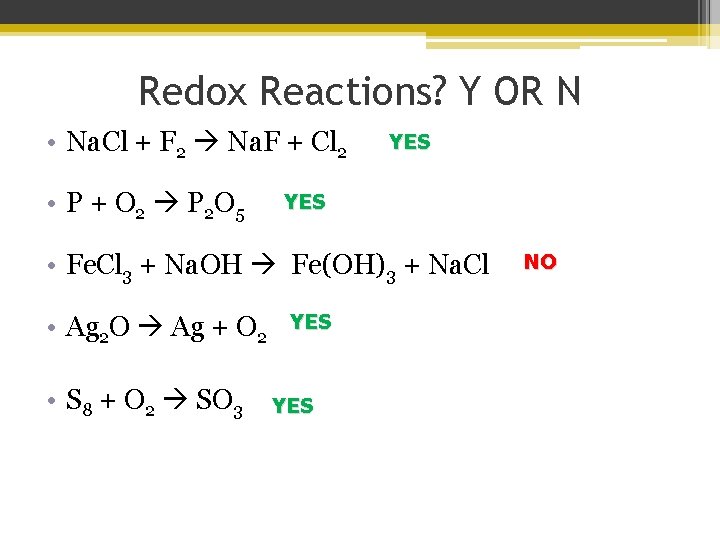
Redox Reactions? Y OR N • Na. Cl + F 2 Na. F + Cl 2 • P + O 2 P 2 O 5 YES • Fe. Cl 3 + Na. OH Fe(OH)3 + Na. Cl • Ag 2 O Ag + O 2 • S 8 + O 2 SO 3 YES NO

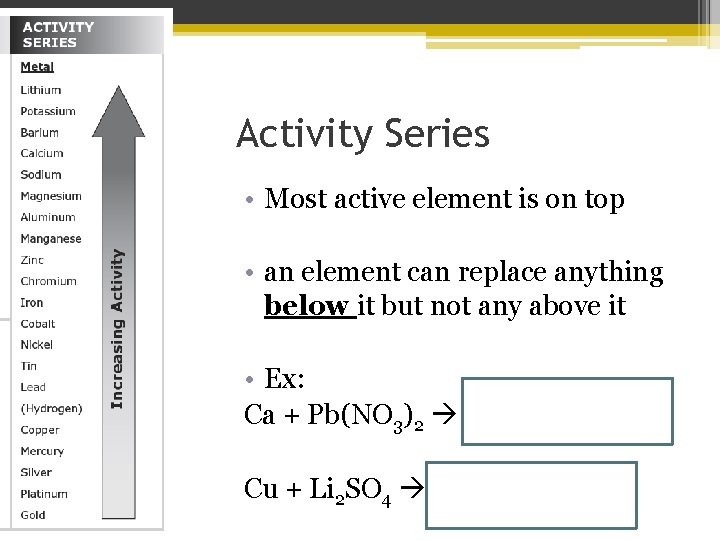
How to determine if a reaction will happen? • We use the Activity Series ▫ Ability of an element to react ▫ The easier it reacts, the higher the activity ▫ List of elements organized from highest to lowest

Activity Series • metals ▫ greater activity, easier to lose electrons ▫ Why? easier to become a cation

Activity Series • Most active element is on top • an element can replace anything below it but not any above it • Ex: Ca + Pb(NO 3)2 Ca(NO 3)2+ Pb Cu + Li 2 SO 4 no reaction

Practice • Zn(s) + HCl(aq) Zn. Cl 2 + H 2 • Br 2 + Fe. Cl 2 no reaction

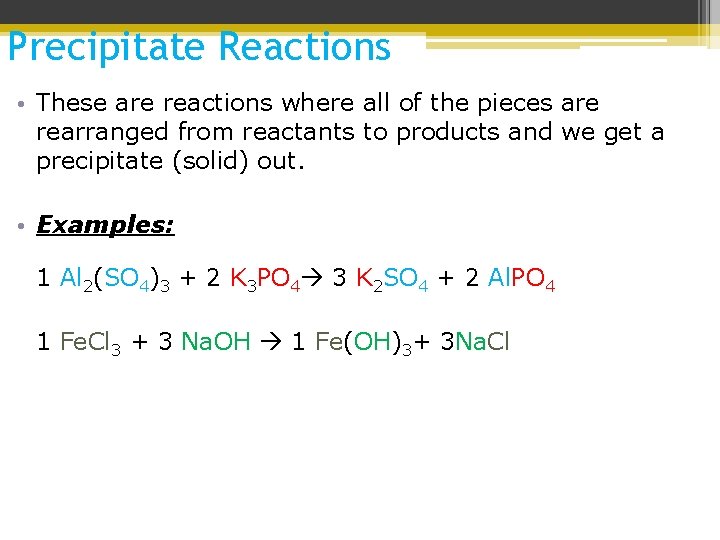
Precipitate Reactions • These are reactions where all of the pieces are rearranged from reactants to products and we get a precipitate (solid) out. 1) Precipitate: solid produced within a solution as a result of a chemical reaction 2) Aqueous solution: a solution where the solvent is water

Precipitate Reactions • These are reactions where all of the pieces are rearranged from reactants to products and we get a precipitate (solid) out. • Examples: 1 Al 2(SO 4)3 + 2 K 3 PO 4 3 K 2 SO 4 + 2 Al. PO 4 1 Fe. Cl 3 + 3 Na. OH 1 Fe(OH)3+ 3 Na. Cl

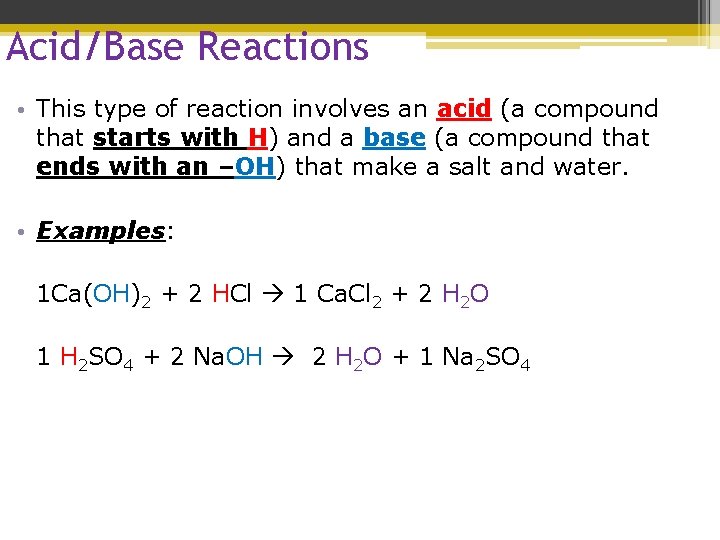
Acid/Base Reactions • This type of reaction involves an acid (a compound that starts with H) and a base (a compound that ends with an –OH) that make a salt and water. • 1. Salt – metal and nonmetal

Acid/Base Reactions • This type of reaction involves an acid (a compound that starts with H) and a base (a compound that ends with an –OH) that make a salt and water. • Examples: 1 Ca(OH)2 + 2 HCl 1 Ca. Cl 2 + 2 H 2 O 1 H 2 SO 4 + 2 Na. OH 2 H 2 O + 1 Na 2 SO 4

Practice H 3 PO 4 + Sr(OH)2 H 2 O + Sr 3(PO 4)2 Na. OH + HCl Na. Cl + H 2 O